Who Is Involved In A Clinical Trial?
Di: Samuel
confirming that the participant understands the difference between receiving a standard of care and participating in research.
This trial involved investigational chemotherapy along with computerized . Healthy volunteers say they participate to help others and to . In this guide we describe the conduct of clinical trials under the CTR in Ireland.
About inspections of clinical trials for human drugs
Supervision of the Conduct of a Clinical Investigation.Clinical trials can lead to life-changing new medicines, but they’re only possible with the help of every person involved. Trial volunteers receive compensation for their time, as well as trial medication and trial-related care at no cost. When research teams ask another organisation for funding, peer review is often part of the funding approval process. The sponsor investigator initiates and conducts a clinical trial – alone or with a team. In some companies, Clinical Project Managers (CPM) may be referred to as Clinical Trial Managers (CTM) or Study Managers. The peer review group includes doctors, other health .
Why Should I Participate in a Clinical Trial?
In this Series paper, we provide a historical perspective on clinical trial research by revisiting the 1948 streptomycin trial for pulmonary tuberculosis, which was the first documented randomised clinical trial in the English language, and . They offer only temporary, condition-specific treatments or procedures.
Clinical Trials Guide
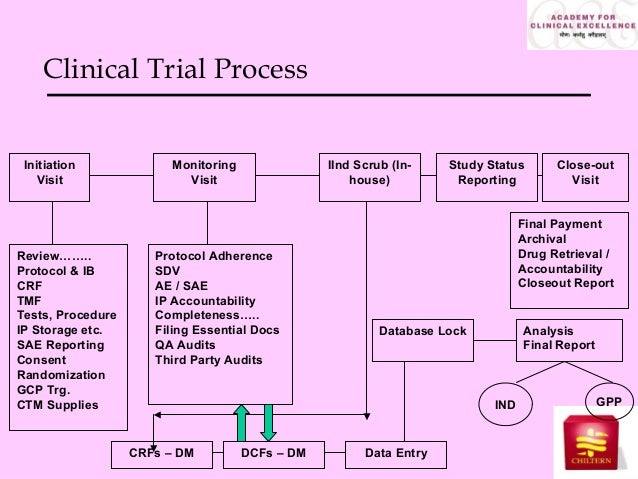
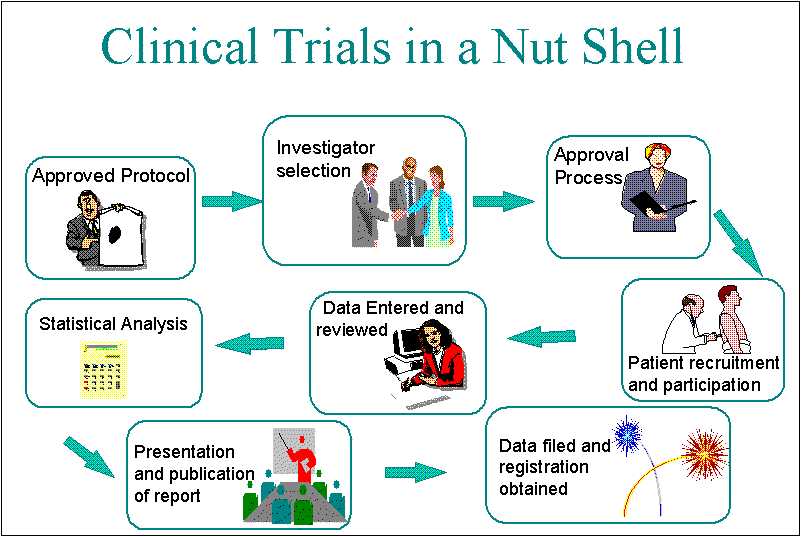
Generally speaking, the purpose of a clinical trial is to collect safety and effectiveness data on a given medical product.A clinical trial is a medical research study that uses participants to: build on current medical knowledge.If new to clinical trials, it is best to get involved in one clinical trial initially and fully understand the processes involved in more detail. It outlines how the trial will be run. Although they may seem intimidating at first, clinical trials can be extremely beneficial no matter what type or stage of cancer you have. another already in use. The first step in the process is to identify a reliable, primary . There are two main types of clinical studies: clinical trials (also called interventional studies) and observational studies. By following best practices such as establishing clear lock criteria, implementing robust data management processes, conducting comprehensive data review and validation, and maintaining regulatory .The Four Phases of Clinical Trials. Compliance with this standard provides public assurance that the rights, safety, and well-being of trial subjects are protected, consistent with the principles that have .Understanding the clinical trial process is a stepping stone toward a deeper appreciation of the efforts and rigorous scrutiny involved in ensuring the safety and efficacy of medical interventions.Nurses advocate for the clinical trial participant by. who will be able to take part (the eligibility criteria) how many people they need to take part. Blinding to avoid bias.
Learn About The People Who Make Clinical Trials Happen
Site Management goes beyond simply monitoring a site. +1-410-502-7683 International.Clinical trials do not provide long-term, comprehensive health care.Based on the trial design and setting, the sponsor may support the investigator by identifying or contracting service providers or personnel to be involved directly in the conduct of the clinical trial (e. Researchers test some of these in a clinical trial with human beings. Take for example, patients participating in a clinical trial for testicular cancer.
Roles and responsibilities
The regulation of clinical trials aims to ensure that the rights, safety and well-being of trial participants are protected and the results of clinical trials are credible. A trial’s ability to provide the intended evidence hinges on appropriate design, background knowledge, trial rationale to sample size, and interim monitoring rules.Since a clinical nurse could influence a patient’s decision about participating in a clinical trial, the nurse should understand what research is and what the particular trial is about. When the goals of economic analysis are .Clinical trials are a fundamental component of medical research and serve as the main route to obtain evidence of the safety and efficacy of treatment before its approval. These documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of Good Clinical Practice and with all applicable regulatory requirements. A typical day in the position includes: Attending tumor board or pathology reviews. The different parties involved in a clinical trial are all possible sources of bias, including: The patient being treated, The clinical staff administering the .FDA Guidance for Industry. (b) The reported trial data are accurate, complete, and verifiable from source documents.
Clinical Trials & the Role of the Oncology Clinical Trials Nurse
Blinding is an important aspect of any trial done in order to avoid and prevent conscious or unconscious bias in the design and execution of a clinical trial. It contains lots of information, including: why they want to do the trial.Several groups with regulatory authority are involved in the conduct of clinical trials in the United States (U.However, the use of placebo may influence the outcome of clinical trials as reported in a systematic review that showed that response rate was on average smaller and dropouts higher for the same antidepressants in placebo-controlled trials compared with head-to-head trials (Salanti et al. Accordingly, we investigated which site-related . Early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is.What is important is to understand the tradeoffs involved when making design decisions and when evaluating cost valued and analyzed from a clinical trial.The project management team is made up of Clinical Project Managers and Program Managers.855-695-4872 Outside of Maryland.
Who’s Who in Clinical Research (2022)
Registration of all interventional trials is a .This will increase the likelihood of a trial that is both timely and cost-effective.Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial.
Participate in a Clinical Trial
Clinical trials are studies intended to discover or verify the effects of one or more investigational medicines.A clinical study involves research using human volunteers (also called participants) that is intended to add to medical knowledge. Check out our 2020 guide below.
Clinical trials
Clinical operations essentially refers to the tasks involved in executing the entire clinical trial, from its design through to its completion.

The Clinical Trial Process: A Step-by-Step Guide
Clinical trials may: compare a new medical approach or product to.
How clinical trials work
Also referred to as an investigator-initiated study (IIS) or investigator-initiated research (IIR), an IIT is a clinical trial in which the investigator conceives the research, develops the protocol, and serves as sponsor investigator. In some cases, the Sponsor also discovered the investigational medicine.Clinical trial monitoring is an important part of trial conduct, and medical monitors are responsible for this aspect of the trial. Psychedelic studies in various stages, enroll both healthy volunteers and individuals with specific mental health conditions. As such, clinical trials pave the way for improved healthcare strategies, contributing significantly to enhancing patient care and outcomes. Ensuring all staff and patients follow trial protocols.
If a sponsor of a CTIMP is a commercial or other non-NHS body, a copy of an insurance or indemnity certificate should normally be included with the REC application as evidence of the cover in place for the potential liability of the sponsor. Below is an up-to-date and searchable compilation of psychedelic clinical trials registered on clinicaltrials.
Guide to Clinical Trials Conducted under the Clinical Trials
According to Ness, clinical trials nurses are critical to preventing delays in study completion and answering the research questions that move science forward. This is a searchable registry and results database of federally and privately supported .
Care of clinical trial participants: What nurses need to know
What pitfalls should I be aware of in general? By working with an experienced CTU and experienced trial methodologists then the risk of pitfalls can be reduced.gov includes both interventional and observational studies. study an approach or product on its own. Later phases (phase 3 and 4) compare the treatment to current standard treatments. If you choose to include your other health care team, you can also be sure that any existing treatments or conditions will not . A list of relevant documents is provided in the References section of this .Deviations from clinical trial protocols and GCP occur commonly in clinical trials.You can be a part of medicine’s future by participating in a trial – either as a healthy volunteer or a patient seeking options that may not be widely available.
Understanding the Benefits of Clinical Trials for Cancer
Clinical trial registration happens when countries seek to improve the transparency of clinical trial research involving nationals of that country, and to be more accountable to the individuals who consent to participate in clinical research, and to better oversee and monitor that research.Just like your auto or health insurance policy, sites, CROs and sponsors generally carry clinical trial insurance to protect themselves or the parties involved in a clinical trial. Health research often involves new medical interventions such as new drugs or devices, new surgical techniques or other treatment procedures.You could be eligible to participate in a psychedelic clinical trial for research.NIH conducts clinical research trials for many diseases and conditions, including cancer, Alzheimer’s disease, allergy and infectious diseases, and neurological disorders.Knowledge of what the pharmaceutical industry emphasizes when assessing trial sites during site selection is sparse.As part of a clinical trial, your study-related medical care is provided by the study team at the location where you’re participating. ClinicalTrials.The goal of clinical trials is to determine if a new test or treatment works and is safe.The mission of the WHO International Clinical Trials Registry Platform is to ensure that a complete view of research is accessible to all those involved in health care decision making. Each phase has a different purpose and helps researchers answer specific questions.
Costing and Cost Analysis in Randomised Trials: Caveat Emptor
Clinical trials in human medicines
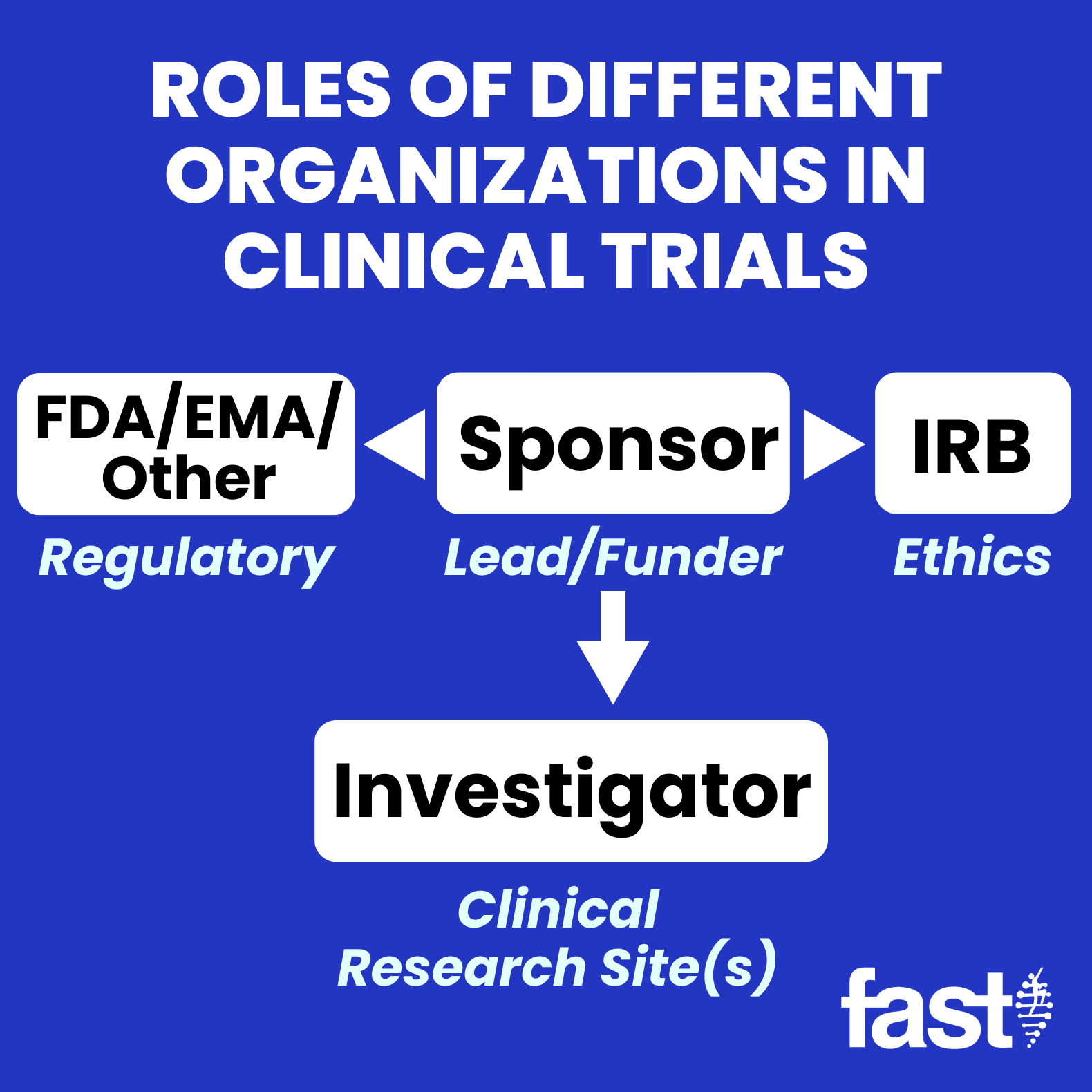
(c) The conduct of the trial is in compliance with the currently approved protocol/amendment (s), with GCP, and with the . Phases of clinical trials. It should be read in conjunction with EU and national legislation, and EC and EMA guidelines. The Sponsor The Sponsor, often a pharmaceutical company, designs and oversees the execution of the study.The HPRA will be actively involved in this initiative through its participation in EMA and HMA.At first glance, it’s abbreviation soup – EMA, FDA, NIHR, OHRP, IRB – but this collection of organizations is responsible for making sure clinical trials are run properly, to protect patients and develop medicines that are safe and effective. It focuses on regular, consistent communication with site stakeholders during the pre-trial, trial, and closeout phases. In a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects ., minors, or patients with severe dementia), the subject should be informed about the trial to the extent compatible with the subject’s understanding and, if capable, the subject .

A better understanding of this issue can improve the collaboration on clinical trials and increase knowledge of how to attract and retain industry-sponsored trials. Today, we are giving investors and clinical research recruiters a brief guide on what to look for in research sites; and what research sites should be doing to make sure they impress. Clinical trials can also look at other aspects of care, such as improving the quality of life for people with chronic illnesses. what the treatments are. Guidance documents describe an agency’s interpretation of or policy on a . assessing the participant’s understanding of the purpose, risks, benefits, and voluntary aspect of participation. Meeting with the care team to review labs . While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. However, common pitfalls include underestimating the . The use of placebo in the control group of a . The clinical operations team works to coordinate all the various departments and teams working on different aspects of a trial, both internal and external, to make sure everything is moving forward and in accordance . Clinical trials are often done in 4 phases. It is up to each country to decide if they want to have .12 When a clinical trial (therapeutic or non-therapeutic) includes subjects who can only be enrolled in the trial with the consent of the subject’s legally acceptable representative (e. Regardless of where they are conducted, all clinical trials included in . In the Code of Federal Regulations (CFR), the two main titles related to clinical trials are Title 45 (Public Welfare) and Title 21 (Food and Drugs).Writing the plan. They have medical and clinical experience and ensure that the trial is conducted properly by .Essential Documents are those documents which individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced. If you have a cancer diagnosis and are weighing your treatment options, consider participating in a clinical trial. However complex it seems, it’s important to understand the role of the agencies involved in . Trials often have research teams and take place in hospitals, medical clinics, doctors‘ offices and universities.Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording, and reporting trials that involve the participation of human subjects.A protocol is the detailed plan of a trial.
A well-executed clinical trial database lock is crucial for ensuring data integrity, reliability, and the success of a study.Another essential element of the clinical trial’s execution and its success is the Site Management Plan. The majority of these instances are technical deviations that do not result in harm to the trial subjects or significantly affect the scientific value of the reported results of the trial. They are accountable of all aspects of a .How clinical trials work. The Principal Investigator The . Recordkeeping and Inspection. providing additional resources to the clinical trial site or qualified personnel). The team interested in doing the trial write a detailed plan (protocol) that everyone involved in the trial will use.
Clinical Research Team
People participate in clinical trials for a variety of reasons. Project managers are the “General Contractors” of clinical research.Researchers, nurses or other health professionals may also be involved in a clinical trial. The research procedure(s) may also carry some risk. Our research clinics located in Austin, Texas; Las Vegas . This section of the guidance clarifies the investigator’s responsibility to supervise the conduct of the clinical investigation and to protect the rights, safety, and welfare of participants in drug and medical device clinical trials. For cost analysis the inconsistency in the application of methods is largely due to the difficult problems that result from the highly skewed cost distribution. To search for other diseases and conditions, you can visit ClinicalTrials.The sponsor is responsible for ensuring that a clinical trial complies with the legislation and GCP.
What Is The Role Of Medical Monitors In Clinical Trials?
This paper shows the scale of global health research and the context in which we frame the subsequent papers in the Series. A group of people who are not involved with the trial should review and approve the protocol. Medical monitors are licensed professionals who act as a consultant and provide their input on a trial’s safety, efficiency, and protocols. test different drug treatments. Here we describe the key goals and . This will improve research transparency and will ultimately strengthen the validity and value of the scientific evidence base. These cases should be documented (for example, in the trial case report form or the trial . a placebo with no active ingredients.

This includes the principal investigator (or study doctor) — the healthcare professional who conducts and takes responsibility for the trial at that location — as well as other clinical study team members.Clinical trials follow a particular timeline, from early, small-scale, phase 1 studies to late-stage, large-scale, phase 3 studies. The purposes of trial monitoring are to verify that: (a) The rights and well-being of human subjects are protected.1 While there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. Including your current health care team may help with receiving comprehensive care. Learn who conducts clinical trials and what’s involved in running one.
- Which Songs Are Progressive House?
- Who Is Circus Baby In Five Nights At Freddy?
- Who You Say I Am Songtext , Who You Say I Am by Hillsong Lyrics
- Which Poker Chips Are The Heaviest?
- Who Is The Ceo Of Mankind Pharma Limited?
- Whirlpool Awe 5105 Waschmittel
- Who Is The Blacklister? – The Blacklist season 5
- Who Was June Carter Cash? | June review
- Who Is The Head Of State Of The Cook Islands?
- Who Is Evelin Villegas Husband Corey Rathgeber?
- Who Restored Paris Is Burning?
- White Lines Neue Folgen , die Krankenhaus-Serie mit Herz und Humor
- Who Is Battlefront? – How To Fix The Error Code 1017 In Star Wars Battlefront 2