When Was Benzene Discovered , August Kekule von Stradonitz
Di: Samuel
Benzene was discovered by: A. Carcinogenesis findings in animals were not reported conclusively until 1979. During a drug induced daze in 1858, Friedrick Kekule stumbled on his theory of the benzene structure and it seemed a simple solution to the mind boggling molecule of Mr 78.Benzene is represented by this symbol, where the circle represents the delocalized electrons, and each corner of the hexagon has a carbon atom with a hydrogen attached. Benzene molecules are a ring of six carbon atoms that are each bonded to one hydrogen atom. The hydrocarbon that we now call benzene was first isolated in 1825 by Michael Faraday from an oily film that deposited from the gas used for lighting.When benzene (C 6 H 6) was first discovered its low hydrogen to carbon ratio (1:1) led chemists to believe it contained double or triple bonds. As a young man in London, Michael Faraday attended science lectures by the great Sir Humphry Davy.

> At that time, private and public buildings were lit by portable gas, which was created by dropping whale or fish oil into a hot furnace. Join / Login >> Class 11 >> Chemistry >> Hydrocarbons >> Aromatic Hydrocarbon >> Benzene was discovered by: | Chemistry Q. The problem is that C-C single and double bonds are different lengths.Discovery of Benzene.
How was benzene was discovered?
Ever since the 1930s debate has raged inside chemistry circles concerning the fundamental electronic structure of benzene. Choose the best answer to complete the sentence. Benzene is naturally found in crude oil.
structure of benzene
Therefore, the correct answer is option A. Benzene is said to be aromatic in nature and the . What’s more, in 1821 he invented the first electric motor, .
Benzene Ring Explained
Benzene also is used to make some types of lubricants, rubbers, dyes, detergents, drugs, explosives and pesticides. Surprisingly, benzene was entirely unreactive toward bromine. It was not until the 1930’s that Kekule’s structure was confirmed by X-ray and electron diffraction. The cardinal reason for the . Each carbon atom in benzene was .39 Å in length and each bond angle being .

Michael Faraday was the first scientist to discover benzene in 1825. =>Benzene, discovered by Faraday, became the starting point in the manufacture of many dyes, perfumes and explosives.In 1834 Eilhardt Mitscherlich made a substance that he realised was the same as that discovered by Faraday but he gave it the name benzene. 2577) has shown that the compound which we now term ‚ benzol,‘ or .Câu hỏi: 14/08/2020 23,646.Michael Faraday discovered benzene 165 years ago in cylinders that had held acetylene gas.Benzene structures throughout history.Benzene, also known as benzol, is an organic chemical compound with the formula C 6 H 6. Benzene is a colourless, volatile liquid with a characteristic sweet odour. It is described as an ‘aromatic’ hydrocarbon; each molecule of benzene is composed of a ring of 6 carbon . A lot of pharmaceuticals are also derived from benzene.August Kekule von Stradonitz (born Sept.Phenol, Ph-OH, or C6H5OH, for example, is formed when an alcohol (-OH) group displaces a hydrogen atom on the benzene ring. Kekule was born into an upper-middle-class family of civil servants and as a schoolboy demonstrated an aptitude for art . 1592 Faraday’s Discovery of Benzene.134 nm) That would mean that the hexagon would be irregular if it had the Kekulé structure, with alternating shorter and longer sides . It was later named by August Kekule, who proposed its structure in 1865. Section: 17-19.Piper and Vane later discovered that this prostaglandin had an effect similar to a known enzyme responsible for the contraction of nonvascular smooth muscle. Crude oil is refined into gasoline by using heat, pressure and chemicals in the refinery to separate the spectrum of petroleum products from crude oil. Patterns of problems. The refining process yields gasoline . He claimed that the idea came to .
Benzene was discovered by:
Home Science Vol.In 1825 he isolated and described benzene.

The electrical industries, together with the industries based upon benzene, constitute overwhelming proof—if proof be needed—of the value of research in pure science.Benzene is a planar molecule (all the atoms lie in one plane), and that would also be true of the Kekulé structure. And ever since the discovery of its unusual stability towards electrophilic addition reactions, chemists tried to come up with a structure that would explain this feature. Because of the aromaticity of benzene, the resulting molecule is planar in shape with each C-C bond being 1.Benzene has a rather sweet smell and in the 19th and early 20th centuries people used the chemical as an aftershave.While benzene was discovered by Michael Faraday in 1825, more than one hundred years before Lonsdale’s work, a century later, scientists still knew only a few things about benzene and its derivatives: they all shared the same six-carbon ring core, had incredible stability, and baffling reactivity. Thus, the classification of TCAs differs from other classes of antidepressant drugs, which are classified based on their mechanism of action. Benzene, which was discovered by Faraday, became the starting point in the manufacture of many dyes, perfumes and explosives. Further studies demonstrated that aspirin minimized some effects of vasodilation response, ultimately leading Vane to consider that aspirin was inhibiting the synthesis of . The gas was compressed and stored in containers for use, but a liquid would condense when the gas was pressurized.
August Kekule von Stradonitz
The Discovery of the Benzene Ring: The discovery of the benzene ring is a tale that revolves around the brilliant mind of Friedrich August Kekulé, a German chemist who lived in the 19th century.Benzene was first discovered by English scientist Michael Faraday in 1825 and he invented this in the illuminating gas.
Aspirin: Turn-of-the-Century Miracle Drug
Click here?to get an answer to your question ️ Benzene was discovered by: Solve Study Textbooks Guides. Benzene was isolated in 1825 by Michael Faraday from the oily residue in the illuminating gas lines in London.As a chemist, Faraday discovered benzene, investigated the clathrate hydrate of chlorine, invented an early form of the Bunsen burner and the system of oxidation numbers, and popularised terminology such as anode, cathode, electrode and ion. Exposure is greater among people who spend significant time in motor vehicles in areas of congested traffic.Benzene’s history resembles that of many another substance discovered, industrially exploited, and then increasingly regulated across the world over the last two centuries of the chemical industry .During the mid to late 1800’s, several possible structures (shown below) were proposed for benzene. Thus benzene, similar to phenol, can be abbreviated Ph-H, or C6H6.
Structure of Benzene (C6H6)
August Kekulé (1829 – 1896) On July 13, 1896, German organic chemist Friedrich August Kekulé passed away.How was Benzene Discovered. He went on to work for Davy and became an influential scientist in his .
Benzene
Benzene ranks in top 20 chemicals because of the production volume and this is used in the industries to make other chemicals that are used for the preparation of .Its discovery and how it got its name.Benzene, which was discovered by Faraday, became the starting point in the manufacture of many dyes, perfumes and explosives.Based on an average benzene concentration of 12.TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an .History of Benzene. Who discovered benzene . From left to right: Claus (1867), Dewar (1867), Ladenburg (1869), Armstrong (1887), Thiele (1899), and Kekulé (1865). Benzene was discovered in the year 1825 by Michael Faraday in illuminating gas.This volume presents an evaluation of the carcinogenicity of benzene, updating with new data the most recent evaluation provided in Volume 100F of the IARC Monographs. Since double and triple bonds rapidly add bromine (Br 2), this reaction was applied to benzene. Faraday experimented with . News & Comment.Structure of Benzene.154 nm) C=C (0.July 2020 0 Tabea Tietz. Basic Structure of Benzene. Best known for his work on electricity and electrochemistry, Faraday proposed the laws of electrolysis. He gave the compound the name benzin. It is commonly used as a solvent in chemical and pharmaceutical industries.5 ppb (40 μg/m 3) in the air and exposure of 1 hour per day, daily benzene intake from driving or riding in a motor vehicle is estimated to be 40 μg. During the end of Kekule’s career he revealed that the structure came to him in a vision after enjoying a glass or two of wine by the fire in his .Since double and triple bonds rapidly add bromine . The two on the right are used today . positive associations with acute lymphocytic leukaemia, chronic lymphocytic leukaemia, multiple myeloma, and non-Hodgkin lymphoma were also recognised at that time. During the mid-1800s, the structure of benzene, a compound extracted from coal tar, confounded chemists. Benzene is carcinogenic, which means it . He discovered that when a permanent magnet was moved in and out of a coil of wire, a current was induced in the coil.Faraday’s Discovery of Benzene | Science.Michael Faraday. He extracted benzene from cylinders of compressed illuminating gas which had been collected from the pyrolysis of whale oil. Solve any question of Hydrocarbons with:-.Surprisingly, benzene was entirely unreactive toward bromine. Recognition of Faraday’s enormous contribution to the sciences has been recognised in .
(PDF) From Poison to Carcinogen: Towards a Global
He proposed that six carbon atoms were joined by a ring of alternating double bonds and single bonds. TCAs have a diverse pharmacological . It is a debate that in recent years has taken on added urgency, because .
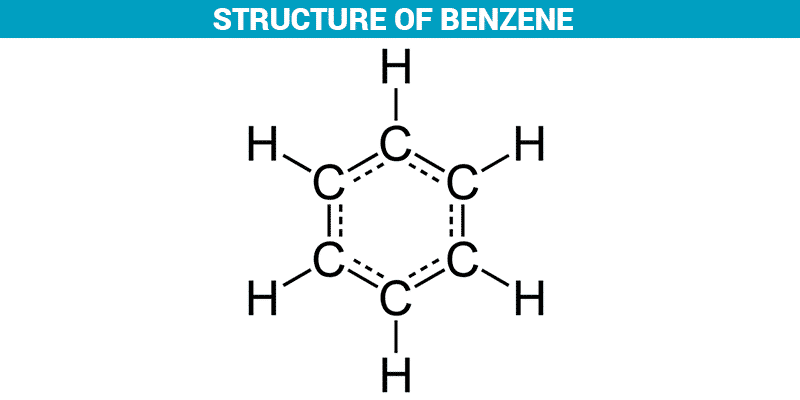
When benzene (C 6 H 6) was first discovered its low hydrogen to carbon ratio (1:1) led chemists to believe it contained double or triple bonds. In fact what you get is -208 kJ mol-1 – not even within distance of the predicted .Since double and triple bonds rapidly add bromine (Br 2), this reaction was applied to benzene. Tạm dịch: Benzen do Faraday khám phá ra đã trở thành điểm khởi đầu trong .
Valisure Detects Benzene in Benzoyl Peroxide
benzene was discovered in 1825, by Faraday, the liquid separating from condensed oil-gas, but Schelenz (Z.
benzene
It is a colorless and flammable liquid with a sweet smell. Faraday ultimately became the first and foremost Fullerian Professor of Chemistry at the Royal .) was a German chemist who established the foundation for the structural theory in organic chemistry. Soon after, the same substance was discovered in coal tar and it quickly became an important chemical commodity and it was found that its molecules actually contained six atoms each of .Michael Faraday first isolated and identified benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name bicarburet of hydrogen. Faraday did some experiments, and discovered that the new compound had equal numbers of carbons and hydrogens, and so named it ‚ carbureted hydrogen ‚. Even today, benzene is used as the starting material for a huge number of chemicals, such as dyes, explosives and many polymers, such as nylon and polystyrene.Applying the same argument to the Kekulé structure for benzene (what might be called cyclohexa-1,3,5-triene), you would expect an enthalpy change of -360 kJ mol-1, because there are exactly three times as many bonds being broken and made as in the cyclohexene case. Industry exploited this discrepancy to discredit the use of animal bioassays as surrogates for human exposure experience. Being one of the world’s leading chemists of his time, he is best known for devising the ring structure of carbon atoms in organic molecules and became the principal founder of the theory of chemical structure. His research on aniline helped lay the basis of the aniline-dye industry, and his research on coal tar laid the groundwork for his student Charles Mansfield’s practical methods for extracting benzene and toluene and . While they understood its .

Benzene, for this very same reason, can be formed from the phenyl group by reattaching the hydrogen back its place of removal. Benzene was discovered by European pharmacists in the 16 th century and the word benzene is basically derived from the word gum benzoin or we can call it benzoin resin which is known as aromatic resin.Benzene, a colorless or light-yellow liquid at room temperature, is a known human carcinogen. Faraday called this newly discovered liquid bicarburet of hydrogen. Benzene, a simple aromatic hydrocarbon, occurs naturally and as a result of human activity, notably as a result of combustion, and it is a high-volume chemical now used . In addition, if benzene is forced to react with bromine . It is an aromatic compound, meaning the ring has alternating double bonds. Trace amounts can be found in cigarette smoke, gasoline, glues, adhesives, cleaning products, and paint strippers. Benzene toxicity in humans has been well . This has an interesting historic background.Michael Faraday first isolated and identified benzene in 1825.The classification of TCAs was based on the three benzene ring molecular core, in part, because the mechanism of action was unknown at the time of discovery. 7, 1829, Darmstadt, Hesse—died July 13, 1896, Bonn, Ger.Benzene-induced cancer in humans was first reported in the late 1920s. He also discovered benzene and other hydrocarbons. Magnets, he knew, were surrounded by forces that could be made visible by the simple expedient of .August Wilhelm von Hofmann (8 April 1818 – 5 May 1892) was a German chemist who made considerable contributions to organic chemistry. In 1833, Eilhard Mitscherlich produced it by distilling benzoic acid (from gum benzoin) and lime. Aromatic compounds have ring-like structures. In the year 1834, a German chemist naming Eilhardt Mitscherlich heated the benzoic acid with lime, which produced .Benzene has been classified as carcinogenic to humans (IARC group 1) since 1979, on the basis of sufficient evidence that it causes leukaemia.Trinitrotoluene (/ ˌ t r aɪ ˌ n aɪ t r oʊ ˈ t ɒ lj u iː n /), more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C 6 H 2 (NO 2) 3 CH 3.
- When Is Hangout Festival 2024?
- Where Can I Watch Hbo For Free?
- When Should I Take Folic Acid?
- Where Are Raleigh Bikes Made? | Where Are Marin Bikes Made? All You Need To Know
- Where Can I Play Pokemon Diamond Version Online For Free?
- Wheels For Wishes New Jersey _ What To Do With Old New York License Plates
- Where Can I Buy Xlm (Xlm)? – Stellar (XLM) Staking at 199%
- When Did Green Arrow Appear In More Fun Comics?
- When Did Meg _ Meg & Dia
- When Is International Solidarity Day Of Azerbaijan?
- When Was The Nigerian Flag First Adopted?
- Where Are Ultra Motorcycles Made?
- Where Did Living Colour Play ‚Cult Of Personality‘?
- When Did ‚Kill Bill‘ Come Out?
- Whatsapp Download Neueste Version