What Is Fda’S New Guidance On Unique Device Identification?
Di: Samuel
Developing a UDI using an FDA-accredited issuing agency’s system.Guidance for Industry.
Unique Device Identification (UDI)
Please indicate Guidance on the Medical Device UDI System in . The final guidance covers recommendations from FDA to help medical device labelers as well as FDA-accredited issuing agencies . This number can be found on the device label or packaging. Its purpose is to associate the use of a device to/on a patient.Unique Identification of Products is Not New 4. Department of Health and Human Services, protects the .com so that you will receive future .10; ( 2) ISO/IEC 15459-4, which is incorporated by reference at § 830.This guidance is being issued consistent with FDA’s good guidance practices regulation (21 CFR 10.A Unique Device Identification (UDI) system is intended to provide single, globally harmonized positive identification of medical devices through distribution and use, requiring the label of devices to bear a globally unique device identifier (to be conveyed by using Automatic Identification and Data Capture and, if applicable, its Human .
IMDRF/UDI WG/N48 FINAL: 2019
This means that UDIs must be printed on all single-use device packaging and labels.Global Unique Device Identification Database (GUDID) . Draft Guidance for Industry and .”1 The UDI System . Document issued on: June 11, 2014. FDA Webinar: Unique Device Identification: Convenience Kits Final Guidance .org) Email: [email protected] Global Unique Device Identification Database (GUDID) contains key device identification information submitted to the FDA about medical devices that have Unique Device Identifiers (UDI). The Global Unique Device Identification Database (GUDID – pronounced Good ID) is a database administered by the FDA as part of the UDI system. Submitting information to .Step 1: Submit Your New GUDID Account Inquiry. The draft of this document was issued on September 24, 2013.Unit of Use (UoU) UDI-DI The UoU UDI-DI is an identifier assigned to an individual medical device. they shall assign to the reprocessed device a new Basic UDI-DI and UDI.The Food and Drug Administration (FDA) is responsible for protecting the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other . The UDI comprises the following components.3 – Definitions.

71, Room 3128 Silver Spring, MD 20993, or by calling 1-800 . This guidance document is being distributed for comment purposes only. The UDI must be presented in two forms on device labels and packages — easily readable plain-text and a scannable automatic identification and . Phone: Australia: 02 6289 8557. The US Food and Drug Administration has published its long-awaited final guidance on form and content requirements for Unique Device Identifier labeling, superseding draft guidance issued in 2016. The UDI Rule also accounts for accessibility in the .The conceptualization of a new Unique Device Identification System established its roots back in 2007, when the idea for a global and singular tracking system piqued the interest of legislators in the United States, who instructed the FDA to develop and oversee new regulations to reduce medical errors, and allow defective medical .

Document issued on June .40 – Use and discontinuation of a device identifier.
Unique Device Identification (UDI)
healthcare supply .
AccessGUDID
The FDA is establishing the unique device identification system to adequately identify devices sold in the U.FDA: Guidance: Unique Device Identification: Direct Marking of Devices; FDA: Unique Device Identification; The FDA, an agency within the U. It allows the unambiguous identification of a specific medical device on the market.A unique device identifier (UDI) must: ( a) Be issued under a system operated by FDA or an FDA-accredited issuing agency; ( b) Conform to each of the following international standards: ( 1) ISO/IEC 15459-2, which is incorporated by reference at § 830.
Devices Guidances
Center for Biologics Evaluation and Research (CBER), Office of Communication, Outreach and Development, 10903 New Hampshire Ave, Bldg.5Mb) The new system will be applied to all medical devices except custom-made and performance study/investigational . Use of International Standard ISO 10993-1, Biological evaluation of medical .FDA Webinar Moderator: Irene Aihie 5-21-19/3:00 pm ET Page 1 .Being unique for each device, the DI component of the UDI can be effectively used by stakeholders to access the GUDID attribute information for that device.1 Unique Device Identification: 2 Direct Marking of Devices. DRAFT GUIDANCE .For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).Division of Industry and Consumer Education Center for Devices and Radiological Health Food and Drug Administration 10903 New Hampshire Ave Silver Spring, MD 20993.- from manufacturing through .based on GS1 global unique numbering and identification systems, barcodes, Electronic Product Code-based RFID, data synchronization, and electronic information exchange.50 – Changes that require use of a new . FDA UNIQUE DEVICE IDENTIFICATION (UDI) Quick Reference Guide to GS1 Identifiers & Barcodes LEARNING THE TERMS FDA UDI GS1 STANDARDS FDA UDI Unique Device Identification GS1 Standards Product Identification Labeler One who applies or modifies the label with intent to put device into commercial distribution Brand . Understanding exceptions, alternatives, and time extensions.The guidance represents the current thinking of FDA on “Unique Device Identification: Policy Regarding Compliance Dates for Class I and Unclassified Devices, Direct Marking, and Global Unique Device Identification Database Requirements for Certain Devices.

The GS1 System of Standards for US FDA Unique Device Identification

SUMMARY: The Food and Drug Administration (FDA, Agency, or we) is announcing the availability of the draft guidance entitled “Select Updates for Unique Device Identification: Policy Regarding Global Unique Device Identification Database . Post: Devices Reforms Taskforce. This guideline is based on the GS1 General Specifications, and was developed using information obtained from all members of the U.Breakthrough Devices Program; Guidance for Industry and Food and Drug Administration Staff CDRH/CBER, September 2023. For questions regarding this document, contact: CDRH: Indira Konduri, udi@fda. to implement the U.A UDI is a unique numeric or alphanumeric code that includes a device identifier, which is specific to a device model, and a production identifier, which includes the current production information for that specific device, such as the lot or batch number, the serial number, manufacturing date, and/or expiration date.gov and noreply@salesforce. Time period covered in this API: 2013 to present. Frequency of API updates: Weekly.For Consult Guidance on UDI System (25May_pub) 923 KB. The FDA has released a long-awaited final guidance on the form and content of its Unique Device Identification (UDI) system, which has been available in draft form for almost five years.This implementation guideline was prepared by GS1 Healthcare US ® to assist suppliers and receivers of medical devices in the U.In collaboration with the National Library of Medicine, the FDA has created a portal, called AccessGUDID, to make device identification information in the GUDID available for everyone–including .
Benefits of a UDI System
Unique Device Identifier (UDI): The UDI is a series of numeric or alphanumeric characters that is created through a globally accepted device identification and coding standard. ACTION: Notice of availability.Rule, establishing the unique device identification system, was published on September 24, 2013 (78 FR 58786).
Unique Device Identification (UDI) System
The guidance document, “Unique Device Identification: Policy Regarding Compliance Dates for Class I and Unclassified Devices, Direct Marking, and Global Unique Device Identification Database Requirements for Certain Devices,” was updated to clarify that FDA does not intend to enforce the GUDID submission requirements under 21 CFR . 2 EXECUTIVE SUMMARY The implementation of a globally harmonized unique device identification (UDI) system will positively impact many aspects of the medical device and healthcare ecosystem by .Unique Device Identification (UDI): Insights and Benefits from a Single UDI System in the International Arena White Paper January 2018 . It requires that the label and each device package of a medical1 IMDRF/UDI WG/N7FINAL:2013 UDI Guidance Unique Device Identification (UDI) of Medical Devices 2 IMDRF/UDI WG/N48 FINAL: 2019 Unique Device Identification system (UDI system) Application Guide – DOCX (12.Global Unique Device Identification Database (GUDID) Changes to the source data: openFDA annotates the original records with special fields and converts the data into JSON, which is a widely used machine readable format. GS1 US also manages the United Nations Standard Products and Services
AccessGUDID
Meeting compliance dates and requirements. 10903 New Hampshire Ave, Bldg. On September 24, 2013 (78 FR 58545), FDA released a document titled “Global Unique Device Identification (GUDID): Draft Guidance for Industry” (the draft guidance).The unique device identification (UDI) is a unique numeric or alphanumeric code related to a medical device. 71, Room 3128, Silver Spring, MD 20993, or by calling 1 -800- 835-4709 or 240-402-7800, by email ocod@fda . FDA UDI Rule using GS1 Standards.Now fully implemented, the Unique Device Identification System offers a range of benefits to industry, the FDA, consumers, health care providers and health care systems by: Allowing more accurate . The guidance represents the current thinking of FDA on “Unique Device Identification System: Form and Content of the Unique Device Identifier (UDI). UDI systems typically use a combination of hardware and software to identify a particular .Unique Device Identification system (UDI system) Application Guide | International Medical Device Regulators Forum (imdrf.

4 Please see the MDCG guidance documents under the ‘UDI Unique Device Identifier . This requires the label of devices to bear a globally unique device identifier. The person responsible for the reprocessing should keep the UDI of the original product as part of the technical documentation and the organisation’s quality management system (QMS) to . The GUDID contains device identification information submitted by device companies to the FDA. Food and Drug Administration Staff . Channeling Johnny Nash and the 1972 classic song, “I Can See Clearly Now,” on July 7, 2021, the Food and Drug Administration issued its final guidance titled, “Unique Device Identification System: Form and Content of the Unique Device Identifier (UDI).The Food and Drug Administration (FDA) is issuing a final rule to establish a system to adequately identify devices through distribution and use. CBER: Office of Communication, Outreach and Development, 1-800-835-4709 or 240-402- 7800.The unique device identification system requirements are being phased in over seven years according to established compliance dates based primarily on device classification. The Unique Device Identification Program 5 Devices 201(h) of FD&C Act . This guidance does not define the term This guidance does not define the term “convenience kit” for other regulatory purposes.Footnotes for this article are available at the end of this page. This rule requires the label of medical devices to include a unique device identifier (UDI), except where the rule provides for an exception or alternative placement. It is assigned in instances when a UDI is not labelled at the level of the device unit of use (e. The Consultation period for this document is from 25 May 2021 to 30 June 2021. International: +612 6289 8557.
FDA issues guidance on UDI direct marking of medical devices
10 – Incorporation by reference.
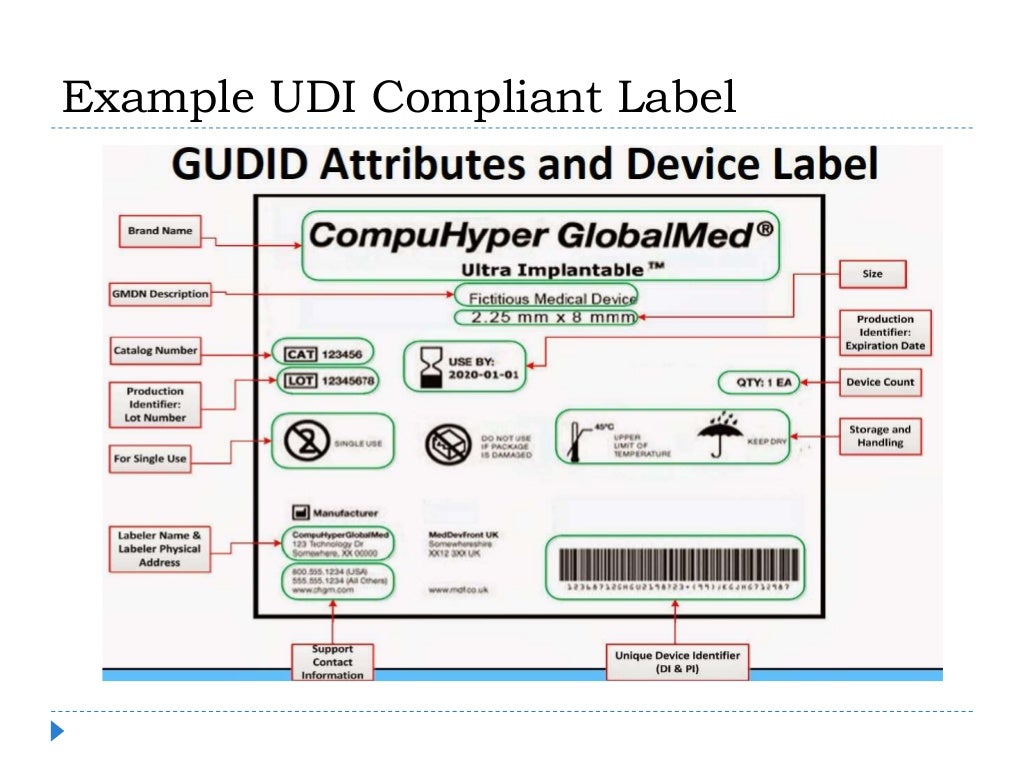
A Unique Device Identification (UDI) system is intended to provide single, globally harmonized positive identification of medical devices through the distribution and use.As its name suggests, unique identifiers are intended to be unique to exactly one device only. It allows for a clear and unambiguous identification of specific devices on the market and facilitates their traceability.
Federal Register :: Unique Device Identification System
Moderator: Irene Aihie Please submit your feedback using the prescribed guidance feedback form via our online UDI enquiry form by 30 June 2021. Therapeutic Goods Administration. Department of Health and Human . These provide access to useful information about .” It does not establish any rights for any person and is not binding on .
eCFR :: 21 CFR Part 830
You should submit .pertaining to the unique device identification system. The UDI is comprised of the UDI-DI and UDI-PI.Start Preamble AGENCY: Food and Drug Administration, HHS. The GUDID contains ONLY the Device Identifier (DI), which serves as the primary key to .20 – Requirements for a unique device identifier. Manufactures and their authorized .What is Saudi-DI? UDI system (Ramz) aims to documenting unique devices codes for medical devices based on accredited international standards, in purpose to allow all stakeholders to identify medical devices information through the unique device identification code that is registered on the system. Additionally, if the device is intended for multiple uses, the UDI must be printed directly onto the device itself.
What is Unique Device Identification
Change your email’s filter settings to allow emails from [email protected], apparatus, implement, machine, contrivance, implant, in vitro .Addition of a new Production Identifier: Donation Identification Number (DIN)-indicates the device is managed by a DIN. several units contained in a plastic bag).
- What Is Discriminant : What is the Discriminant?
- What Is My Sanford Chart 6179930?
- What Is Germany Phone Code 49?
- What Is Mass Effect 2 Storyline?
- What Is An X Match In Family Tree Dna
- What Is Aladin? : Aladdin (2019)
- What Is Arabic Language : Languages of Somalia
- What Is Amaranth? | Amaranth kochen: So geht die Zubereitung
- What Is An Oral Sex – Safer Sex (Safe Sex)
- What Is ‚Indian Camp‘ About? – Indian Camp Summary
- What Is Google Meet – Google Meet
- What Is Microsoft 365? , Microsoft 365 for Business Plans—Free Trial
- What Is Costco Membership? – Is a Costco Membership Really Worth It?