What Are The Principles Of A Clinical Trial?
Di: Samuel
PRINCIPLE recruited participants through this website and also through GP practices . There are precautions researchers can take – in the planning, implementation and follow-up of studies – to protect these participants in research. However, it is important to remember the individuals who volunteer to participate in research.Principles of clinical trial/research.1 These guidelines focus on design, planning, management, conduct and regulation of clinical trials involving human participants. The benefits of conducting trials should outweigh the risks.
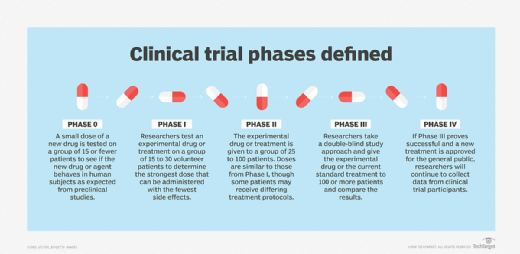
All clinical trials should be conducted in accordance with ethical principles, sound scientific evidence and clear detailed protocols.A clinical guideline applies to all patients with a particular condition, but there will be times when the recommendations are not appropriate for a particular patient. the primary goal of conducting clinical trials in developing countries should be to address the health needs of the host population.NIH Definition of a Clinical Trial. In compliance with the ALCOA+ principles, we help make sure that your data is always complete, consistent, and accurate, while stored in a durable format that is accessible to authorized personnel at all times. Preclinical studies using animals to study the potential of a therapeutic drug or strategy are important steps before translation to clinical trials. However, it is only in far more recent times, since World War II, that concepts such as Beauchamp and Childress’s four principles of beneficence (providing benefits to persons and balancing those against risks and costs fairly), non-maleficence (the obligation to .2 Physical security. The doctor-researcher has to serve both the roles and at times the zeal of an investigator has the potential to cloud the morality of the physician inside.Overview Avoiding errors, collecting data that is fit-for-purpose, and reducing patient burden are just a few of the many benefits of applying Quality by Design (QbD)-an approach that focuses resources on the errors that matter to decision making during a trial. “They supplement PhRMA’s Principles on Conduct of Clinical Trials and Communication of Clinical Trial Results which were strengthened in 2004 and again in . Keywords: p-value, confidence intervals, intent-to-treat, missing data, multiplicity, subgroup analyses, causation. Treatment effects are efficiently isolated by controlling for bias and .Clinical research has been around, in one form or another, since the biblical era. CTTI has built a suite of resources-including recommendations for monitoring, recommendations for . The rights, safety and well-being of trial participants are of paramount importance and these should be preserved by obtaining informed consent . Compliance with this standard provides public assurance that the rights, safety and wellbeing of trial subjects are protected and that clinical-trial data are .ly understands the importance of data integrity in clinical trials and will help you ensure your data’s quality, security, and integrity. This is majorly carried out by collecting the data and analyzing it to derive conclusions. The Scientifi c Group was charged with reviewing and formu-lating principles for clinical evalua tion of drug products, whether new or already marketed, including considerations for new indications or dosage forms for marketed products and new combination products. (c) The conduct of the trial is in compliance with the currently approved protocol/amendment (s), with GCP, and with the .

This principle is referred to as “community-based participatory research”, or CBPR. The purposes of trial monitoring are to verify that: (a) The rights and well-being of human subjects are protected. A trial’s ability to provide the intended evidence hinges on appropriate design, background knowledge, trial rationale to sample size, and interim monitoring rules.The ICH guideline ‚General considerations for clinical studies‘ is intended to describe internationally accepted principles and practices in the design and conduct of clinical studies that will facilitate acceptance of data and results by regulatory authorities, provide guidance on the consideration of quality in the design and conduct of clinical studies .2 SCOPE OF THE GUIDELINES 1. Trial design and registration: Clinical trials are carefully designed; the protocol 1 for conducting the trial and the statistical analysis plan (SAP) detailing the planned data analyses are developed well before the first participant is enrolled.
Quality By Design
The ethics of global clinical trials
This chapter provides an overview of the key potential benefits and risks of data sharing and sets forth the guiding principles (as evolved from the Framework document; see Box 2-1) that informed the committee’s thinking as it considered the issues presented throughout the . These recent initiatives will go a long way in improving quality of clinical trials. Trial protocols provide the background and rationale for conducting a study, highlighting specific research questions that are . The objective of clinical trials is to establish the effect of an intervention. therapeutic strategies: psychotherapeutic and behavioural therapies.In these cases, sponsors remain responsible to conduct the trial in compliance with the protocol and with principles of good clinical practice (Clinical Trials Regulation Art 47, ICH E6(R2) section 5.E6 (R2) Good clinical practice. Trial protocols are documents that describe the objectives, design, methodology, statistical considerations and aspects related to the organization of clinical trials.During his visit he noticed my thesis on the history of the clinical trial lying on my desk. There are various types of clinical trials that are . Multicenter clinical trials provide greater evidence on the generalizability of an intervention than single center trials. Healthcare and other professionals are expected to take our clinical guidelines fully into account when exercising their professional judgement. The protocol represents the means by which a hypothesis will be tested. However, despite these commitments, recent .“The Principles demonstrate the long-held commitment of PhRMA member companies to responsible sharing of clinical trial data,” said PhRMA President and CEO John Castellani. Clinical trials are the main route to obtain this required evidence.
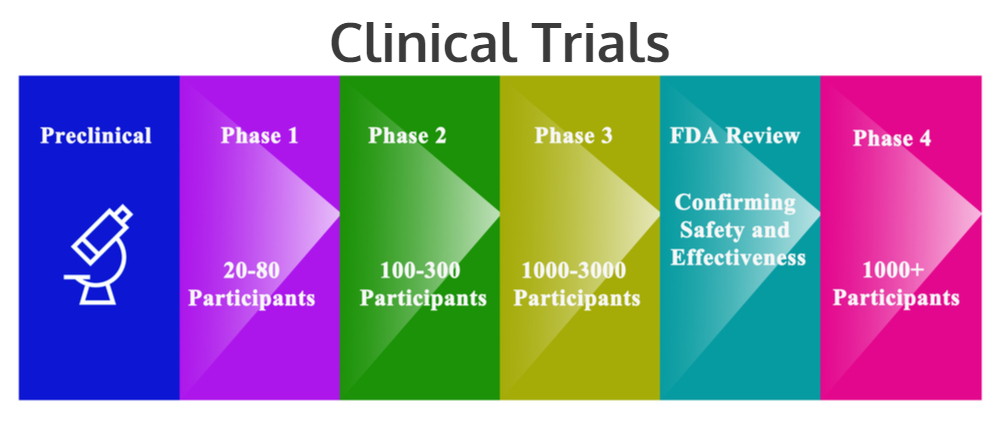
Fundamental clinical trial design issues are discussed.The principles described in The Belmont Report laid a foundation for regulations regarding the ethics of human subjects research in the United States.Clinical trials should be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and that are consistent with GCP and the applicable regulatory requirement(s).,c• ~ation forbetterheakh ICH E6(R3) GCP Principles • Clinical trials should be designed to protect the rights, safety and well-being of participants and assure the reliability of results. (b) The reported trial data are accurate, complete, and verifiable from source documents. Guiding Principles for Sharing Clinical Trial Data. However, evidence has shown that poor quality in the design and conduct of these studies has not only impeded clinical translation but also led to significant waste of valuable .Clinical trials in Singapore are regulated under the Health Products Act and Medicines Act and their subsidiary legislations.
Quality of clinical trials: A moving target
ALCOA+ and Data Integrity in Clinical Trials
A trial’s ability to provide the intended evidence hinges on appropriate design, background knowledge, trial rationale to sample size, and interim monitoring rules.1747: James Lind and Scurvy Trial.Clinical trials study a range of interventions, including: pharmaceutical interventions: experimental drugs, cells and other biological products, vaccines. In his capacity as editor of the Journal of Chronic Diseases, he invited me to prepare an article based on the thesis, and this was published not long after (Bull JP 1959. The historical development of clinical therapeutic trials. 1 – 4 Dr Lind (1716-94), whilst working as a surgeon on a ship, was appalled by the high mortality of scurvy amongst the sailors. Ethics are the moral values of human behavior and the principles which govern these values. Before any treatment is approved and offered to patients in the general population, rigorous evidence of its safety and efficacy must be shown.Data sharing is essential for promoting scientific discoveries and informed decision-making in clinical practice.

A clinical trial protocol is a written document that provides a detailed description of the rationale for the trial, the hypothesis to be tested, the overall design, and the methods to be used in carrying out the trial and in analyzing its results.
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
Journal of Chronic Diseases 10:218248).
Unblinding in Randomized Controlled Trials: A Research Ethics Case
Clinical trials are a fundamental component of medical research.
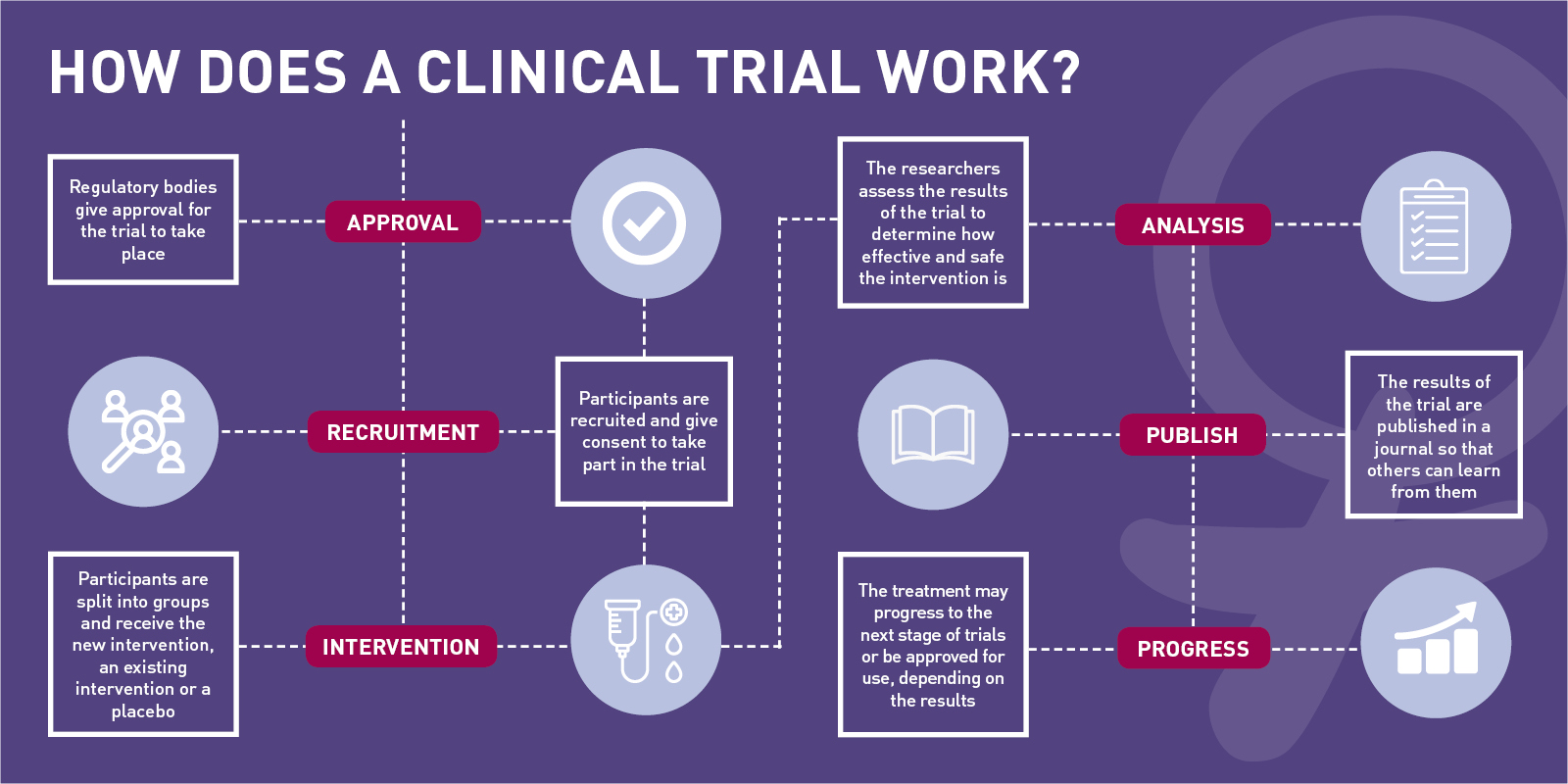
small or large ways from trial to trial but their validity is universal.PRINCIPLE is a UK-wide clinical study from the University of Oxford to find COVID-19 treatments for recovery at home., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to . They apply to trials designed to generate information on the efficacy or safety of medicines. • Clinical trial designs and processes should be proportionate to the risks inherent in the trial and the importance of the data being collected. The system of regulation requires that Principal Investigators (PI) conducting clinical trials to obtain both ethics and regulatory approvals before initiating a clinical trial. The ethical aspects of a clinical trial cannot be separated from the scientific . The extent of security measures depends on the criticality of the data. James Lind is considered the first physician to have conducted a controlled clinical trial of the modern era. Derenzo and Moss ( 2006) commented as follows: Each study component has an ethical aspect. The protocol and the SAP constitute some of the most important metadata of the trial. Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [2,3].
Protocol Development and Preparation for a Clinical Trial
Overview of comments received on draft ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials (EMA/ CHMP / ICH /436221/2017) First published: 26/04/2018 Last updated: 26/04/2018 Reference Number: EMA/744060/2017.The subsequent UK Medicines for Human Use (Clinical Trials) Regulations became law in 2004. Legal background [NEW] From 31 January 2025 onwards only the Clinical Trials Regulation (EU) 536/2014 (CTR) and its Delegated Acts will apply, as laid down in Article 98 thereof.
Regulations: Good Clinical Practice and Clinical Trials
It aims to provide a unified standard for the ICH regions to facilitate . He planned a comparative trial of the most promising cure for scurvy. In this article, we present some general principles of good clinical . We are looking for medicines that can help people with COVID-19 symptoms get better quickly and stop them needing to go to hospital. However, the guidance does not . The regulations are intended to protect the rights, safety and wellbeing of research participants and to simplify and harmonise regulatory processes.Good clinical practice (GCP) is defined as a “standard for the design, conduct, performance, monitoring, auditing, recording, analyzing and reporting of clinical trials.Clinical Trials as Topic / standards*.
Ethics in clinical trials
disease detection and treatment methods: new ways to detect and treat disease, diagnostic or screening tests. On the other hand, the rights, integrity, and confidentially of trial .1 Protection of clinical trial subjects The principles and practices concerning protection of trial subjects are stated in the
Guiding Principles for Sharing Clinical Trial Data
In this review, we provide an overview of the ethical foundations of trial design, trial oversight, and the process of obtaining approval of a therapeutic, from its pre-clinical . Other strengths include a larger sample size, conduct across different settings, greater diversity in participants, the development of protocols that receive input from multiple research teams, and ethical .115 following principles, taken together, capture thenecessary qualities of a well-planned, well-run and 116 clinically relevant trial. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects.
HANDBOOK FOR GOOD CLINICAL RESEARCH PRACTICE (GCP)
Trial risk vs trial benefit Before a trial is initiated, foreseeable risks and inconveniences should be weighed against the anticipated benefit for the . Computerised systems, servers, communication infrastructure and media containing clinical trial data should be protected against physical damage, unauthorised physical access, and unavailability. Although blinding is generally viewed as an effective method by which to eliminate bias, blinding does also pose some inherent limitations, and it behooves clinicians and researchers to be aware of such . The situation becomes challenging for a doctor when he assumes the role of researcher. 22 Among other effects, these principles obligate biomedical researchers to maximize benefits to the participants, within the constraints of the scientific aims of the study, while minimizing harm.convened a Scientifi c Group on Principles for Clinical Evaluation of Drugs.

98 KB – PDF) View. “CBPR is really the focus on everyone’s mind, but it is very difficult to achieve”, Alfano said.also that clinical trial data are likely to be credible and meaningful. During the course of a .E9 Statistical Principles for Clinical Trials September 1998.Clinical equipoise is a cornerstone of such ethical conduct. They do not repeat the ethical principles that underpin sound and ethical research, which are
Guidance for Good Randomized Clinical Trials
Blinding remains under-utilized, particularly in non-pharmaceutical clinical trials, but is often highly feasible through simple measures.Guidance for sponsors – Application for transition of clinical trials from the Clinical Trials Directive to the Clinical Trials Regulation – Version 3 – March 2024 1 1. During good clinical practice (GCP) inspections of commercial as well as academic trials, an increasing amount of deviations from GCP standards have .principles established in this guideline may also be applied to other clinical investigations (e. In 2013, PhRMA/EFPIA recognised the importance of data sharing and supported initiatives to enhance clinical trial data transparency and promote scientific advancements.The FDA has partnered with Duke University for Clinical Trials Transformation Initiative, which will conduct research projects on design principles, data quality and quantity including monitoring, study start-up, and adverse event reporting. It provides assurance that the obtained data and reported results are credible and accurate. radiotherapy, psychotherapy, surgery, medical devices and alternative therapies).regulations, preambles, human subject protection, good clinical practice, research, investigation, trial, investigator, IRB, institutional review board
ICH GCP
Other fundamental ethical principles include respect for persons, beneficence, and justice (see Turner, 2010 ). 23 In relation to .
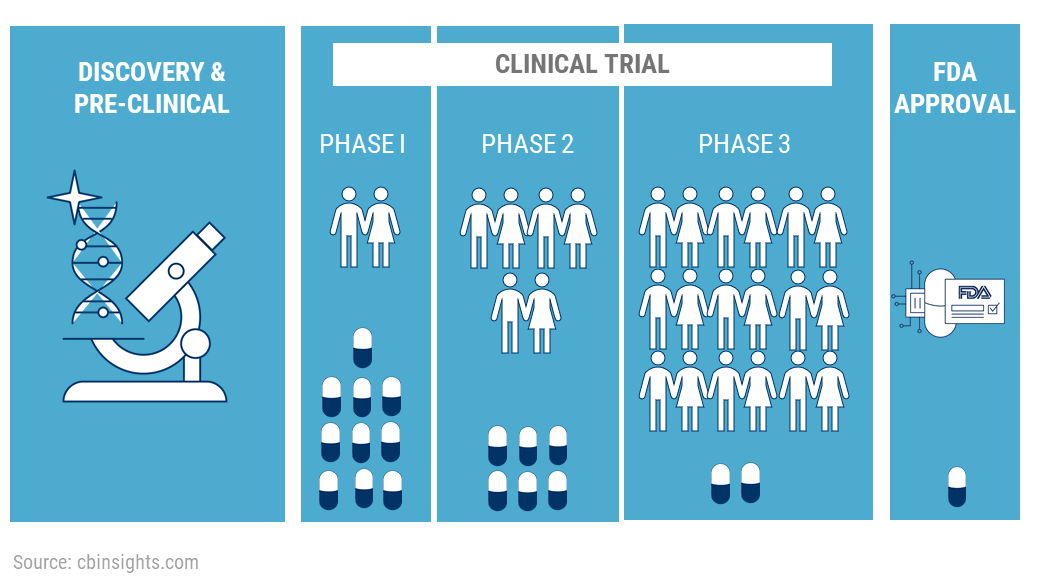
A research study in which one or more human subjects are prospectively assigned prospectively assigned The term prospectively assigned refers to a pre-defined process (e. The efficacy and safety of medicinal products should be demonstrated by clinical trials that follow the guidance in E6 Good Clinical . Good RCTs are118 designed to produce scientifically . The methods and approaches needed to achieve these qualities will differ in 117 .
Key concepts of clinical trials: a narrative review
NICE clinical guidelines
English (EN) (927.Although conducting a well-designed clinical trial may appear straightforward, it is founded on rigorous methodology and oversight governed by key ethical principles. GENERAL PRINCIPLES 2.Clinical trials are a fundamental component of medical research and serve as the main route to obtain evidence of the safety and efficacy of treatment before its approval.Clinical research advances the understanding of science and promotes human health.Good clinical practice (GCP) is an international ethical and scientific quality standard for designing, recording and reporting trials that involve the participation of human subjects. Ethical approval is provided by the Institutional Review .
- What Are Terraria Mods? _ Terraria: Die besten Mods im Überblick
- What Are The Rcts Of Lamotrigine?
- What Are Some Popular Japanese Baby Names?
- What Are The Natural Remedies For Whooping Cough?
- What Are German Chocolate Bars?
- What Are The Best Free Screensaver Apps For Windows?
- What Are The 3 Parts Of A Magazine Article?
- What Do You Say To St Jude? _ How to Publish Your Prayer or Thanks
- What Are Some Famous German Landmarks In Berlin?
- What Are Plastics Properties? , Acrylonitrile Butadiene Styrene (ABS Plastic): Uses, Properties