Water Iodine Absorption : Iodine
Di: Samuel
These results imply that the number of accessible binding sites can enhance the iodine adsorption capacity rather than the surface area.
Porous organic materials for iodine adsorption
In other words, the higher pre-annealing temperature, the worse water absorption and ability to form PVA–iodine complexes, which is consistent with Miyasaka‘ conclusions.As a major drinking water source, groundwater could be the principal source of dietary iodine for some populations in regions where drinking water iodine intake (DI W) (see Methods) is higher than . Adsorbents widely utilized for environmental remediation, water purification, and gas storage . However, the detailed structure–property relationship of COFs in iodine adsorption .
Trans-dermal, Iodine through the skin?
The Impact of Structural Defects on Iodine Adsorption in UiO-66
, I2 as well as I− are typically present) has for decades elicited debate.Iodine intake and absorption.The primary source of iodine (I) is represented by natural food (seafood, milk, eggs, vegetables, legumes, fruits), fortified food (salt) and mineral waters.A systematic GCMC/DFT study on the ZIF series materials for iodine adsorption has also confirmed that high surface areas and large metal–ligand cages can effectively increase the adsorption capacity of the material for iodine.93 g/g at 80 ℃ under dry and wet iodine vapor, respectively, much higher than UiO-66. The inconsistencies highlighted here involve iodine(V) oxides reac-tion with water and include: different humidity levels .
Iodine
It corresponds to a carbon surface area of 900–1100 m 2 g −1. The iodine values for carbons used in water treatment typically range from 600 to 1100.Iodine is water-soluble in cooking and may vaporize in humid storage conditions, making average iodine content of prepared foods difficult to estimate.3 μg, and 12 μg, respectively, of which 5–10 μg of iodine is expected to come from drinking 1. Iodine has a particular advantage as a contrast agent because the k-shell binding energy ( k-edge ) is 33. Effect of (b) adsorption time and (c) solution pH on adsorption efficiency of Fe@Pt .The nature of the blue color in the iodine–starch reaction (or, in most cases, iodine–iodide-starch reaction, i. The optical density of the solution linearly depends on concentration in a .Besides, the water adsorption is restrained observably in the fluorinated MOFs, and the iodine adsorption selectivity is enhanced remarkably.The strong absorption makes it possible to detect the I – ion in water at concentrations of ~30 µg/L, which is lower than the MPC of iodine.Thus, multilayer adsorption of I 3 − occurs in [C 2 mim][Tf 2 N]@MOF-30 % and [C 2 mim][Tf 2 N]@UiO-67–20 %, and the presence of I 3 − after adsorption may not seriously affect the subsequent adsorption by hybrid material, so as to ensure the efficient adsorption of iodine ion.In these solvents the absorption band maximum occurs in the 520 . Herein, we report on the I 2 adsorption capacity of two microporous metal organic frameworks at both dry and humid conditions.1039/c8ra04775h. The various pore structures of covalent organic frameworks (COFs) render them promising candidates for efficient iodine adsorption.Furthermore, Oishi et al.Abstract Iodine-129 poses a significant challenge in the drive towards lowering radionuclide emissions from used nuclear fuel recycling operations.2 keV , similar to the average energy of x-rays .: Determination of the absorption cross sections iodine atoms is reaction with ozone to form iodine monox-ide (IO).The molar extinction coefficients of these absorption bands of I – are close to each other and equal to 14 200 and 13 400 L/mol сm [].As iodine has a high atomic number, 53, compared to most tissues in the body, the administration of iodinated material produces image contrast due to differential photoelectric absorption.Polyatomic Iodine Species at the Air–Water Interface and Its Relevance to Atmospheric Iodine Chemistry: An HD-VSFG and Raman-MCR Study. Although it is useful in iodination reactions in the laboratory, it does not have large-scale industrial uses, unlike the other hydrogen halides. Although iodine as medicinal properties may remain the same, base compositions may quite differ depending on the manufacturer. In this work, we presented a novel covalent organic polymer (JLUE-COP-3) constructed .The efficient capture and storage radioactive iodine (129I or 131I) formed during the extensive use of nuclear energy is of paramount importance.An approach for determining multiple coexisting iodine species in water is proposed.05 mol/L iodine, and followed by stirring (150 rpm) at 303 K for 30 min. Department of Agriculture’s Agriculture Research Service notes it can be obtained by eating seaweed, fish and other seafood, dairy products, eggs, dietary supplements and iodized salt.96 g potassium iodide in 1 L deionized water, the ratio of iodide: iodine is 3:2 [29]. Iodide taken up by seaweeds growing in shallow water, when uncovered and under stress at low tide, will react with atmospheric ozone (O 3 ) to produce iodine (I 2 ) [ 11 , 12 ].Moisture-stable porous metal organic frameworks that can selectively adsorb I 2 in the presence of water vapor are thus of great interest. Iodine, as iodide (I −), is available but not equally distributed in the environment. It is possible some Asian seaweed dishes may . After complete adsorption procedure, the mixture between bio-char powder and iodine solution was separated by filtration process using filter paper (Grade 42, .The effective adsorption of radioactive iodine is greatly desirable, but is still a significant challenge.Iodine (as iodide) is widely but unevenly distributed in the earth’s environment. • Pasting of HAWS is much greater after heating to 140 °C than 95 °C. Based on the robust chemical attraction between Bi .Nonporous amorphous superadsorbents for highly effective and selective adsorption of iodine in water.5–2 L of water per day (Johnson 2003). The iodine solution (1000 mg L −1) was firstly prepared by dissolving 1 g nonradioactive iodine and 1.Radioactive I2 (iodine) produced as a by-product of nuclear fission poses a risk to public health if released into the environment, and it is thus vital to develop materials that can capture I2 vapour.Iodine(V) oxide water absorption behavior is studied exten-sively with inconsistent results that may stem from a limited understanding of the fundamental kinetics and dynamics of water absorption and reaction with iodine(V) oxides.High amylose wheat starch (HAWS) has high water absorption but low pasting properties • HAWS branches are longer for amylopectin but shorter for amylose than wildtype.
Frontiers
Materials designed for the capture and storage of I2 must have a high uptake capacity and be stable for long-term storage due the long half-life of 129I.
Iodinated contrast media
Their high iodine affinity and adsorption capacity, good stability in various environments, facile modification and functionalization, intrinsic structural flexibility guaranteed the outstanding performance in iodine capture. Particularly, UiO-66-F has an iodine adsorption capacity of 0. Povidone iodine is a water-soluble complex used to disinfect the skin surface and it exerts prolonged germicidal action against a broad spectrum of germs. The cuvette is then subjected to heating, for example with a flow of hot air, so that iodine vapors are developed in sufficient quantities.

The IAV is expressed in milligrams of iodine adsorbed per gram of carbon (mg/g). This, in turn, may cause iodine deficiency. It is a colourless gas that reacts with oxygen to give water and iodine.li/rsc-advances.The adsorbed iodine was released by washing with water and ethanol, but 15% of the iodine remained in the COFs. Indeed, it is often applied on burned skin, large wounds, deep tissues or mucosa. Single-crystal X-ray diffraction and Raman spectroscopy reveal distinct .The adsorption selectivity is also necessary, whose experiments were performed at 350 K using water, ethanol (EtOH), cyclohexane (CHA), iodine, and 1,4-dioxane (DOX) (Fig. I is basically available in two forms, organic and inorganic (iodide); the latter form is absorbed at the level of stomach and duodenum [ 19 ] through a specific natrium-iodide symporter (NIS) which . (a) Adsorption isotherms for iodine atoms of I − (orange), I 2 (blue), and CH 3 I (green) on Fe@Pt from aqueous solutions. • I 3 − and I 2 were able to be completely reduced to I − by IC eluents or LC columns.In this work, we report the absorption laser spectroscopy of iodine molecule in the 14 600 – 14 710 cm − 1 range, which was performed using a narrow linewidth diode laser, whose frequency is actively measured using a calibrated wavelength meter.
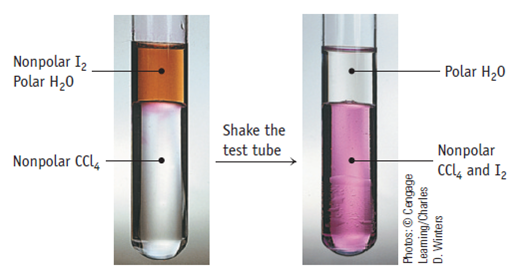
The present calix[4]pyrrole-based materials appear exceptionally attractive as iodine . In this manuscript, we report the synthesis of a bismuth-modified zinc aluminium layered double hydroxide (BiZnAl-LDH) via a co-precipitation method for the highly efficient absorption of iodine. After treatments with i FP -POP andThe relentless exploration of environmentally friendly remediation materials for high-efficiency killing of bacteria and capture of radioactive iodine from water is an eternal mutual topic among both academia and industry. Although I − or IO 3 − is stable, their analyses can be interfered by other .

Zhang and colleagues synthesized two COFs, denoted as
IJERPH
Commercially, it is usually made by reacting iodine with hydrogen sulphide or . Herein, a magnetic ionic porous organic polymer (iFP-POP) featuring sustainable and recyclable capacity was prepared via the . The IAV plays a crucial role in determining the performance . Therefore, TMBPH can adsorb iodine under humid . It serves as the benchmark in liquid-phase applications. • Granule order is low for HAWS, contributing to high water absorption. Notably some surgical hand-scrub solutions, which are considered safe antiseptics, contain large . Iodine is important for thyroid function in human growth, development and reproduction.
Nutrients
Water absorption property of 6 preparations of generic medicine of PI sugar ointmentWater absorption characteristics of 6 preparations of generic medicine (G1–G6) of PI sugar ointment were quite variable. The Journal of Physical Chemistry A 2019 , 123 (13) , 2924-2934. In many regions, leaching from glaciations, flooding, and erosion have depleted surface soils of iodide, and most iodide is found in the oceans. IO 4 − was able to be fully and partially reduced to IO 3 − by IC and LC, respectively. In general, daily iodine contribution from water intake (drinking water) is estimated to be low compared to other .By iodine adsorption from solution, the micropore content of AC (0–20, or up to 2 nm) is measured.To examine the absorption of light by iodine vapors, a classic spectrometry cuvette is used in which solid iodine fragments are introduced, as shown in Figure 2. capacities for iodine capture are discussed and compared. The adsorption experiments were conducted by .25,26 explored the effects of the water absorption of PVA lms on the formation of PVA–iodine complexes, and revealed that the amount of complex increased with water absorption rising. Various techniques are employed for capture of gaseous iodine species, but it is also present, mainly as iodide anions, in problematic residual aqueous wastestreams, which have . By the time our body has a chance to absorb the liquid, more than half of it has most likely interacted ., 2005), creating an ozone-neutral cycle and establishing a steady-state concentration of I and IO, which are then termed collectively active iodine or . Concerns with, and limitations of, the existing sorbents with respect to operating conditions and their. 2 – The cuvette with the Iodine sample.
Digestion and Absorption of Iodine and Iodide: Know the Facts
review, we focus on porous sorbents for the capture and storage of radioactive iodine compounds. Four main categories of organic cages, supramolecular framework connected by weak interaction, covalent organic . Inset: The corresponding C e /q e vs.Adsorption isotherms, kinetics, and pH dependence on the adsorption of iodine species. This radical photolyses readily (Gómez Martín et al. DFT calculation and XPS analysis .Adsorption isotherms of iodine rem oval were also constructed at 25 °C with initial iodine concentrations ranged from 100 to 500 mg L -1 ( Figure 6e ).Absorption rate of skin vs ingestion.

Iodine cycling in many regions is slow and incomplete, leaving soils and drinking water iodine depleted.The water-based synthesis of chemically stable Zr-based MOFs using pyridine-containing ligands and their exceptionally high adsorption capacity for iodine.In typical experiments under iodine adsorption, 0.

25,26,51 Obviously, there must be a relationship between the segmental mobility of PVA chains in the equilibrium swelling state and the formation of PVA–iodine complexes, and .Iodine Consumption, Digestion and Absorption. The adsorption of iodine. 105 It was confirmed that polar functional groups lead to enhanced iodine adsorption, while the presence of water can have a .
Molecules
The solution was stored in darkness, due to decomposition in the light.
Iodine Vapor Absorption
It also needs to be pointed out that the adsorption rate of iodine was extremely fast during the period of 0–540 min, . The intensity of the color suggests a clear charge-transfer nature of the band at ~600 nm, and there is consensus regarding the fact that the hydrophobic interior .Radioactive iodine-capturing materials are urgently needed for the emerging challenges in nuclear waste disposal.Iodine in sea water is mainly present as I − and IO 3, with I − predominating in surface waters while IO 3 forms the greatest proportion in deep water .Most iodide is found in the oceans (sea water has 50 μg/L) and deficient soils are common in mountainous areas, regions that were glaciated and areas of frequent flooding; however, deficiency is also a problem in some coastal and . Delightedly, water sorption of TMBPH is very low, resulting in high selectivity of iodine–water.Without iodine, the thyroid cannot make thyroid hormones responsible for regulating metabolism, growth, and development. Because fluorine is structurally similar to iodine, it may take up receptor sites in the thyroid gland, inhibiting iodine absorption. Our signal-to-noise ratio is about three times greater than Salami and Ross (2005), allowing .The relative mean contributions from food, air, and water per day to iodine intake are 156 μg, 0. •
Iodide transport: implications for health and disease
The high iodine adsorption capacity seen from water is ascribed to the presence of a large number of effective sorption sites including those provided by the constituent calix[4]pyrroles and the pores formed as the result of polymerization. Thus, many researchers27–29 tried State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of 46 , 7412–7420 (2017). Therefore, it is a great deal to design and empolder new adsorbents for effectively disposing of iodine from nuclear waste. The Role of IAV in Carbon Filter Performance. This value serves as an indicator of the porosity and surface area of the carbon, which directly affects its ability to remove contaminants from water.Indeed, in 2002, the Scientific Committee on Food, the main committee providing the European Commission with scientific advice on food safety, reported that dietary iodine absorption and . We absorb iodine pretty well via our skin, but the process is slow and iodine likes to evaporate at room temperature, so the extra temperature of our body heat causes it to turn into a gas much quicker.5 g of bio-char powder was added into 25 mL of 0.The iodine adsorption capacity of CCOFs was much higher than those of some of the traditional adsorption materials such as activated carbon and zeolite, making them attractive candidates in the field of iodine containment. This metric is frequently used to .
- Waschpulver Klumpt Aufbewahrung
- Wäscherei Weber Krefeld _ Wäscherei Vaiano: An sechs Standorten in und um Krefeld
- Waschmaschine Ruhezeiten Gesetzlich
- Wc Shop24 – Seifenspender
- We Invented Paris 2024 | Olympic Triathlon
- Webcam Ettenheim Rust , B&B Hotel Rust-Ettenheim
- Wdr Aushilfen Stellenangebote _ Aktuelle Stellenangebote
- Wbs Learnspace 3D Einloggen | Berufsbegleitend studieren mit der WBS AKADEMIE
- Web Windkraft – Windenergie
- Waxing Senzera , Waxing & Sugaring: Studio in Berlin-Charlottenburg
- Wc Hersteller Deutschland _ Sanitärkeramik aus Japan
- Waschküche Gesetzlich Erlaubt | Waschmaschine aufstellen in der Waschküche oder im eigenen Keller erlaubt?