Volume Pressure Temperature , Gas Laws
Di: Samuel
We recommend using the latest version of Chrome, Firefox, Safari, or Edge. Since work is energy, it has units of Joules (where 1 J = 1 kg ⋅ . 1: Now imagine that we have a container with a piston that we can use to compress the gas inside. Both the increase in pressure and the decrease in temperature cause the volume of the gas sample to decrease.6: The Combined Gas Law- Pressure, Volume, and Temperature is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Calculate the pressure exerted by the gas if it is compressed to a volume of 0.We decide to compress the box down to 1 m 3, but we don’t change the overall . The ideal gas law is easy to remember and apply in solving problems, as long as you use the . Heat represents the agitation energy of the elementary particles that compose matter: atomic molecules and electrons.For example, when the pressure of 1.
Gas Laws
In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state.Hayden Cox (Furman University, Class of 2018) 2.An action that changes the temperature, pressure, or concentrations of reactants in a system at equilibrium stimulates a response that partially offsets the change while a new equilibrium condition is established (2).A gas occupies a volume of 0.The Combined Gas Law relates pressure, volume, and temperature of a gas.12 The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm.To calculate pressure increase with temperature, you can use the ideal gas law, which states that P1 / T1 = P2 / T2, where P1 is the initial pressure, T1 is the initial temperature, plumbing issues, replace old pipes, and ensure there are no obstructions in the water supply line. Check Your Learning.The relationship for these variables, \[P V = n R T\] where R is known as the gas constant, is called the ideal gas law or equation of state. dmaslach@gmail.
What is the ideal gas law? (article)
The Ideal Gas Law is conveniently rearranged to look this way, with the multiplication signs omitted: PV = nRT.00 atm and a temperature of 40 o C is set with a pressure relief valve set to open at a pressure of 10.2 shows how the solubility of a gas in water goes down as the temperature is raised.Calculated simplified in order to be able to calculate mass, volume, pressure, or temperature using various units. As we have seen from kinetic theory, .The properties of an ideal gas are all summarized in one formula of the form: p \cdot V = n \cdot R \cdot T p ⋅ V = n ⋅ R ⋅ T. This decrease in the solubility of oxygen as temperature goes up is one of the reasons cold water fish like trout can not live in warm water.
The Ideal Gas Law
The initial pressure is 100 kPa (or 10 5 Pa if we use scientific notation), and the volume of the container equals 2 m 3.

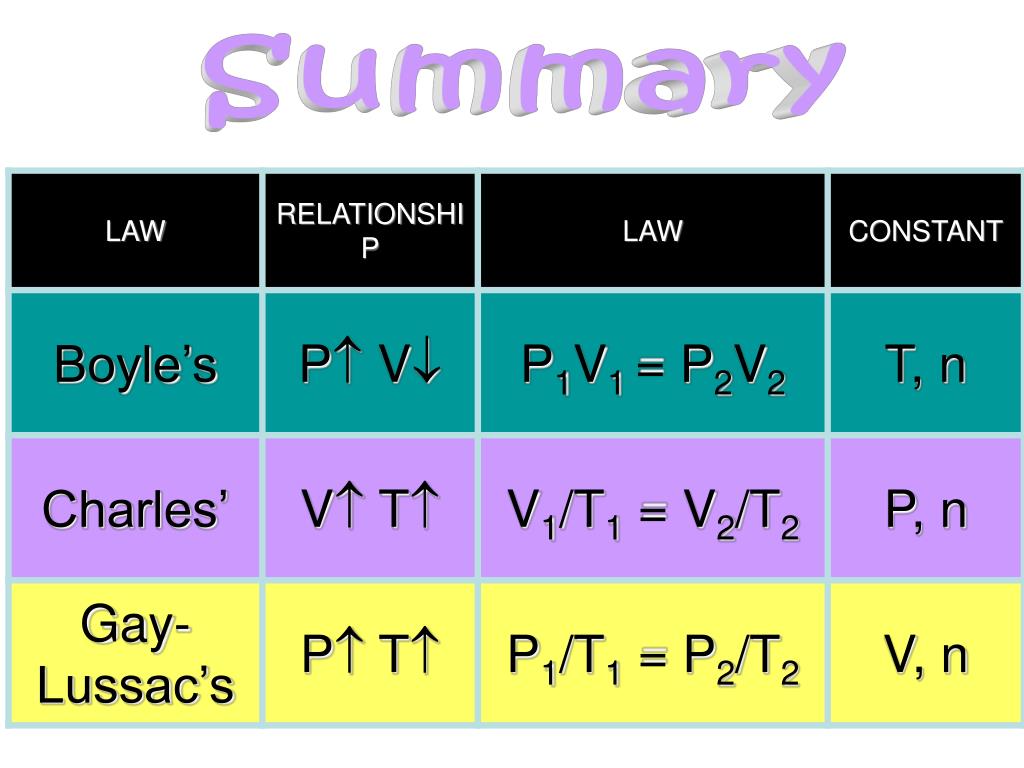
P1V1 = P2V2 = constant. The value of R R in different units of P .work = w = − P external × Δ V. 2: Solubility of several gasses in water. (a) Here are actual data from a typical experiment conducted by Boyle.Systems at equilibrium can be disturbed by changes to temperature, concentration, and, in some cases, volume and pressure; volume and pressure changes will disturb equilibrium if the number of moles of gas is different on the reactant and product sides of the reaction. The line stops at 111 K because .This way, the user can get accurate values of entropy, enthalpy, steam quality and more for a selected pressure, temperature or both, either in the wet steam, saturated or superheated regions of the T-S, P-V or any other diagram.
Hydrogen Conversion Calculator
Steam Tables Online Calculator
50 m³ at a pressure of 100 Pa.The Ideal Gas Law. Putting these together leaves us with the following equation: P1 × V1 T1 × n1 = P2 × V2 T2 × n2. Therefore, V1, old volume = 5.It is summarized in the statement now known as Boyle’s law: The volume of a given amount of gas held at constant temperature is inversely proportional to the pressure under which it is measured.

2 mL at 30 °C and 452 torr.15 °C, which is called absolute zero because no lower temperature is possible (unless, of course you can come up with a negative volumes, but you cannot).
Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Step 3: Think about your result. This relationship, illustrated in part (b) in Figure 5. Use the contact tab above if you have any questions or issues. P1, old pressure = 2 atm. With everything tied together by the ideal gas law, one variable can always be described as dependent on the other two.The extrapolated temperature corresponding to zero volume at constant pressure and amount is -273. As with the other gas laws, we can also say that (P × V) (T × n) is equal to a constant. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous . 13 will describe numerous types of phase diagrams for multicomponent systems.08206 L atm mol –1 K –1 and 8. Volume of a Gas Sample. Their physical definition, is however more complex than it seems. The initial volume and . The SI base units specifies certain units for various types of quantities, based on seven fundamental units.15 K (0 °C) and 1 atm (101.Clearly, from the model you can see that as the volume of gas decreased, its molecules become more crowded and collide more often with the walls of the container. It is the result of a long historical evolution.
Pressure-Volume Diagrams
00atm × 308 K = 1.3145 kPa L mol –1 K –1. V2, new volume = 2. Includes 53 different calculations.com ©2024 Dan Maslach . The system’s response to these disturbances is described by Le Chatelier’s principle: .38 × 10 − 23 J K.5*10^4 Pa (at constant temperature) its new volume can be calculated using the useful form of .

The ratio of volume to temperature is constant when pressure is constant.Avogadro’s Law shows that volume or pressure is directly proportional to the number of moles of gas.The specific volume, an intensive property, is the system’s volume per unit mass.We can state Charles’s and Gay-Lussac’s findings in simple terms: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in kelvins). If the temperature is in kelvin, volume and temperature are directly proportional.A pressure tank containing chlorine gas at a pressure of 2.relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple gas laws standard conditions of temperature and pressure (STP) 273.
Volume (thermodynamics)
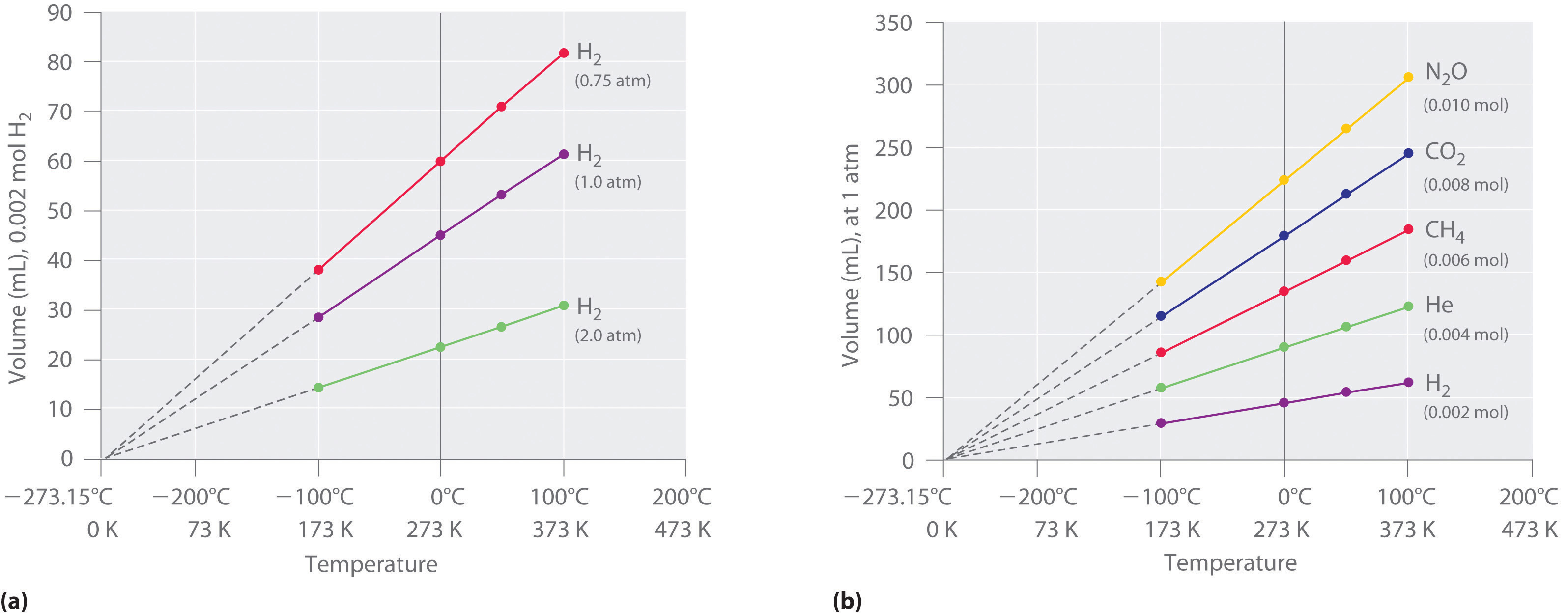
Since both changes are relatively small, the volume does not . Vocabulary Kelvin temperature: The absolute temperature scale where \(0 \: \text{K}\) is the theoretical absence of all thermal energy (no molecular motion). At what temperature will the relief valve open? A gas is in a sealed cylinder at 25 o C with a piston which can expand or contract to change the volume.4: Properties of Matter – Mass, Length, Volume and Temperature is shared under a CC BY-NC-SA 4.Because PV is always a constant, we can equate the two states and write: P1V1 = P2V2 P 1 V 1 = P 2 V 2.The units used to express pressure, volume, and temperature will determine the proper form of the gas constant as required by dimensional analysis, the most commonly encountered values being 0. ( T constant) V. The combined gas law relates .A system can be described by three thermodynamic variables — pressure, volume, and temperature.We can use Boyle’s law in several ways, so let’s take a look at some examples: Imagine that we have an elastic container that holds a gas.At the same temperature, the \(A\) molecules generate a pressure \(P_A\), and the \(B\) molecules independently generate a pressure \(P_B\). A sample of oxygen, O 2, occupies 32. The volume (V) occupied by n moles of any gas has a pressure (P) at temperature (T) in Kelvin. Well, maybe it’s only two variables. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Why was this calculator made? This Online Steam Calculator was made after many other free online steam properties .325 kPa) standard molar volume volume of 1 mole of gas at STP, approximately 22.Real gases tend to deviate from ideal gases at high pressures and low temperatures, as the attractive forces between molecules and the volume of gas molecules becomes significant. Equations displayed for easy reference. The value of R R can be calculated by: R = PV nT R = P V n T, where n n is the quantity of gas in a mole, T T is the temperature in kelvin, P P is the pressure that can be in various units, and V V is the volume that can be in various units.We have developed macroscopic definitions of pressure and temperature.PDF 02-05-2019. If we substitute in the variable R for the constant, the equation becomes: P × V T × n = R.
Boyle’s Law Calculator
Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature.Properties of the gaseous state predicted by the ideal gas law are within 5% for gases under ordinary . Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the . volume-temperature (constant pressure) The volume of a gas is directly proportional to its temperature when pressure is constant.Online calculator with Saturated Steam Table by Pressure.0 license and was authored, remixed, and/or curated by LibreTexts. Since these pressures are generated independently, we conclude that the total pressure is the sum of the two partial pressures—which is, of course, just Dalton’s law of partial pressures. This chapter describes pressure–volume and pressure–temperature phase diagrams for a single substance, and Chap.If we want to use N number of molecules instead of n moles , we can write the ideal gas law as, P V = N k B T. For convenience we set the degrees on the Kelvin scale to the size of the degree on the . Xethyleneglycol = 1 − 0. Determine the pressure of the gas at a volume . To find any of these values, simply enter the other ones into the ideal gas law calculator. Step 1: Since this solution is only comprised of water and ethylene glycol, we can easily calculate the mole fraction of ethylene glycol in this solution by subtracting water’s mole fraction from 1. 2: Plots of Boyle’s Data.0 mL at a pressure of 13.
Particles in gases
00 atm × 308 K = 1. You are told that, initially, the pressure in the container is 765 mm Hg and the volume is 1. Where P is the pressure of the gas, V is the volume taken up by the gas, T is the temperature of the gas, N is the number of molecules in the gas, and k B is Boltzmann’s constant, k B = 1.This answer supports our expectation from Charles’s law, namely, that raising the gas temperature (from 283 K to 303 K) at a constant pressure will yield an increase in its volume (from 0.4 L for gases behaving ideallyVolume-temperature data for a 1-mole sample of methane gas at 1 atm are listed and graphed in Figure 9.

This relationship between pressure and volume is known as Boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure.The equation: PV = nRT P V = n R T is called the ideal gas law.A phase diagram is a two-dimensional map showing which phase or phases are able to exist in an equilibrium state under given conditions. 3 is often referred to as Charles’s law and is stated mathematically as.
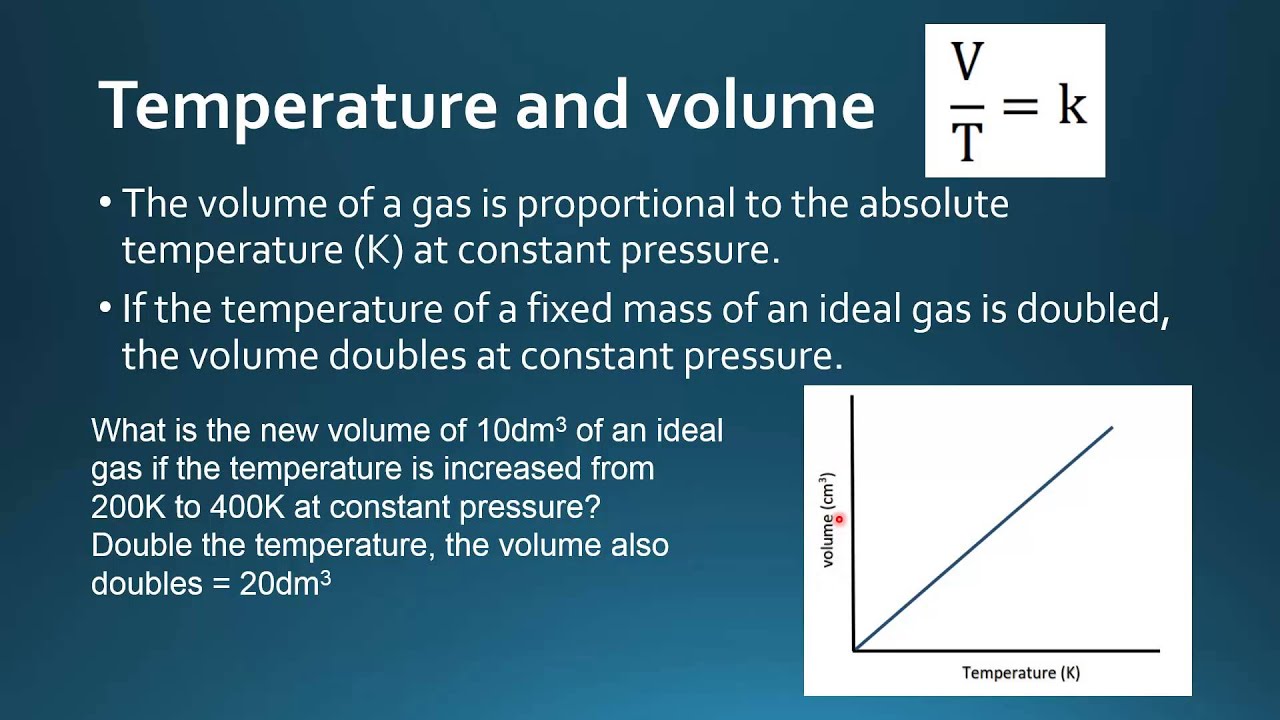
For example, if you want to calculate the volume of 40 moles . There are other gas laws that relate any two physical properties of a gas. This relationship is known as Charles‘ law or Gay-Lussac’s law. As a result, the new pressure inside the container should be higher than the old pressure. The sample of gas in [link] has a volume of 15.
Gases Intro
The constant can be evaluated provided that the gas . where: T T – Temperature of the gas, measured in kelvins.00L × 273 K 1.00 L × 273 K 1.314 kPa L mol –1 K –1. Assume that the temperature and mass of the gas . Hence, Le Châ telier ’s principle states that any change to a system at equilibrium will adjust to compensate for that change. Thermophysical properties of air: Boiling temperature (at 1 bara): 78. of sulfur dioxide gas changes from 5.6 *10^3 Pa to 1. Pressure, temperature and heat are quantities used in everyday life, especially in meteorology.The pressure a gas would create if it occupied the total volume available is called the gas’s partial pressure. Pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer.
Mass, Length, Volume and Temperature
where P external is the external pressure (as opposed to the pressure of the gas in the system) and Δ V is the change in the volume of the gas, which can be calculated from the initial and final volume of the gas: Δ V = V final − V initial. If two or more gases are mixed, they will come to thermal equilibrium as a result of collisions between molecules; the process is analogous to heat conduction as described in the chapter on temperature and heat.
The Ideal Gas Law is a single equation which relates the pressure, volume, temperature, and number of moles of an ideal gas.Calculate the vapor pressure of pure ethylene glycol at this temperature.Thermal properties of air at different temperatures – density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusivity and more.
- Vorerkrankungen Einfach Erklärt
- Volkswagen Automodelle , Aktuelle Aktionen und Angebote
- Vonovia Kamen _ Vonovia in Dortmund-Dorstfeld: Neue Ideen für das Quartier
- Vollmacht Für Gewerbeanmeldung Vorlage
- Vollverklebungen Automotive : Autovollverklebung
- Vorausabtretung Beispiele _ Folgen der Abtretung
- Vorbehaltsgut Voraussetzungen Pdf
- Vollstationären Pflege Vereinbarung
- Vom Kindergartenkind Zum Schulkind
- Volkswagen Polo Versicherung Kosten
- Vollstreckungsankündigung Erhalten Anwalt
- Vorhangschienen Zum Kleben , Gardinenstange ohne Bohren anbringen
- Volvo Xc70 D5 Erfahrungen | V70 D5 Partikelfilter nachrüsten?
