U235 Vs U238 Nuclear , Urânio-235
Di: Samuel
U235 U238 Pu239 Thermal range -1. 233 U decays via alpha decay into 229 Th with a half-life of 159 200 years. The nuclei of atoms consist of protons and neutrons, with the number of protons determining the element (e.Nuclear reactions also often involve γ rays, and some nuclei decay by electron capture.70% uranium 235.
The activity concentration of . A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
El Uranio: un elemento químico radioactivo
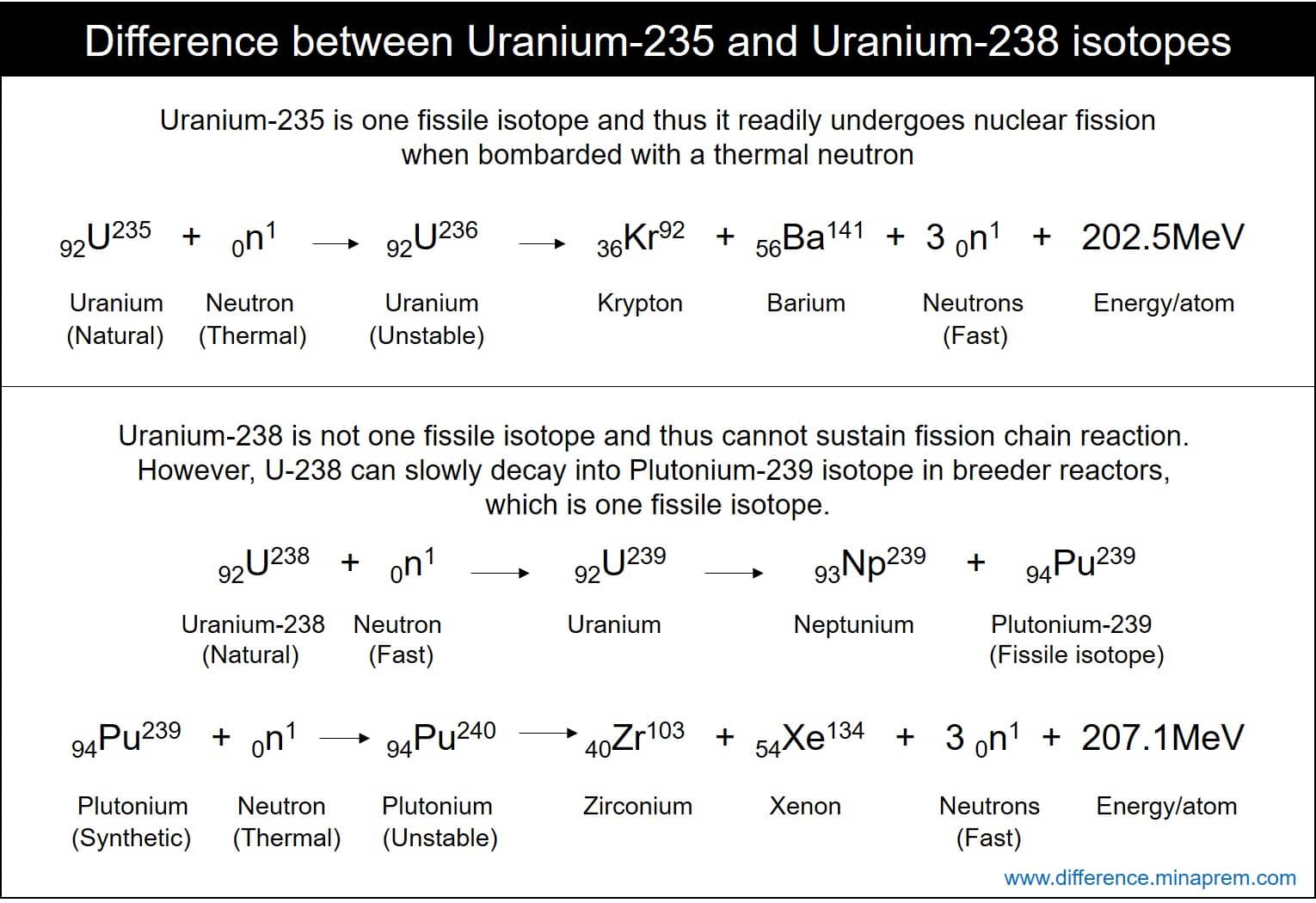
Pore-water U reduction is believed to commence at the Fe +2 –Fe +3 redox boundary, though the rate of U reduction and accumulation in sediments is enhanced in the presence of metal- and sulfate-reducing bacteria (Bargar et al.Uranio 235 vs Uranio 238 El uranio es un elemento de metal pesado que abunda en el núcleo de la tierra. All nuclear decay processes follow first-order .Spectral Variation of Neutron Cross Sections: U -238. nuclear weapons usually use plutonium-239 in the primary stage, but the jacket or tamper secondary stage, which is compressed by the primary nuclear explosion often uses HEU with enrichment between 40% and 80% along with the fusion fuel lithium deuteride.7 Fast range 0. Its specific activity is ~0. O urânio é um elemento de metal pesado abundante no núcleo da Terra. Some substances undergo radioactive decay series, proceeding through multiple decays before ending in a stable isotope.7 wt% of U235, which is fissile material while the re maining 99.This video explains about radioactivity and the reason why Uranium 238 is more stable than Uranium 235.Uranium Management and Policy.Uranium-235 is one fissile isotope, thus it can sustain nuclear fission chain reaction with thermal neutron. Uranium-238 is not a fissile isotope and thus cannot sustain chain reaction with any neutron.I know U-238 is more stable than U-235, because it is an even-even nucleus. That allows a chain-reaction in which neutrons from one fission activate the next fission, etc.
Uranium Radiation Properties
That is why crossing the 4. In general, uranium-235 and uranium-234 pose a greater radiological health risk than uranium-238 because they have much shorter half-lives, decay more quickly, and are thus more radioactive. The Kovarex enrichment process is a method for reprocessing uranium in a centrifuge. Su reactividad nuclear es la razón principal para calentar el núcleo de la tierra y conducir a fenómenos como la deriva continental.
Urânio-235
Fuel burnup defines energy . Applications for HALEU are today limited to research reactors and medical isotope production.People who live near federal government facilities that made or tested nuclear weapons, or facilities that mine or process uranium ore or enrich uranium for reactor fuel, may have increased exposure to uranium. High-assay low-enriched uranium (HALEU) is defined as uranium enriched to greater than 5% and less than 20% of the U-235 isotope.
Isotopes of uranium
Different isotopes of the same element have the same . Much smaller thermal increase in capture (~10X) Unresolved resonance range begins at ~10 keV Threshold fission at ~1 MeV (~10% of total fission rate in a fast reactor) 8. The nuclei of uranium 235 and 238 are, along with those of . But Pu-239 is itself fissile, so you can use it in a reactor (or bomb), rather than waiting around for it to turn into U-235.Nuclear Fission.
Nuclear Fuel Facts: Uranium
En las aplicaciones actuales, el uranio se utiliza en reactores nucleares y armas militares. The other fissile nuclei are U233, Pu239, and Pu241. Though small amounts of highly .72% do urânio natural. The stability of a nucleus to fission has to do with its binding . Thus there is usually a series of decays until the . Uranium that is depleted (U-235) is used in industrial settings (i.Starting from the recently released JENDL-5 library comprising the nuclear data and consistently their covariance ( Nuclear Data Center Japan Atomic Energy Agency, 2022 ), this study aims at testing U-235, Pu-239 and U-238 data above the resolved energy region. Uranium is a silvery-white metallic chemical element in the periodic table, with atomic number 92. For the secondary of a large nuclear weapon, the higher critical mass of less . 236 U decays via alpha decay to 232 Th with a half .El U-235 es capaz de sostener una reacción en cadena de la fisión nuclear.
why does 235 work in atom bombs and power plants and 238 doesn’t? u/BearItChooChoo didn’t quite get it right.5% U235 threshold is significant.
Distinction between Fissionable, Fissile and Fertile
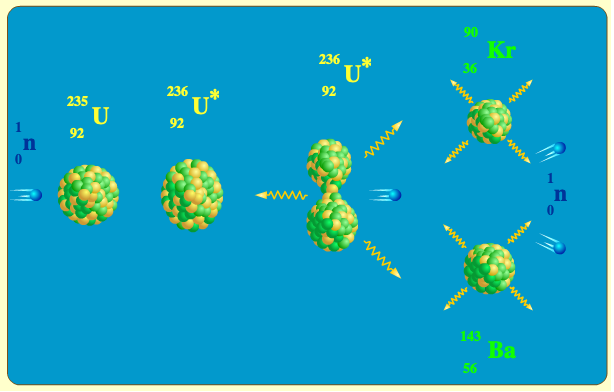
It is used in the production of nuclear fuels and the atomic bomb. U238 can also be used in the production of plutonium, which is another element commonly used in nuclear weapons. The nucleus of the U-235 atom comprises 92 protons and 143 neutrons (92 + 143 = 235). The presence of U-236 and Pu-239/240 in depleted uranium has been confirmed by analyses of penetrators collected during the UNEP-led mission to Kosovo in November 2000. 233 U occasionally decays by spontaneous fission with a very low probability of 0. As Wikipedia mentions, producing high grade Pu-239 like that is not free of problems, since it easily absorbs a neutron .The current fleet of nuclear reactors runs primarily on uranium fuel enriched up to 5% uranium-235 (U-235). Uranium-235 is much rarer than its counterpart, uranium-238.The making of nuclear fuel.28% of natural uranium, is the most common isotope of uranium in nature. Only neutron with energy larger than 1., carbon has 6 protons, while uranium has 92) and the number of neutrons determining the isotope of that element. If the mass of the fragments is equal to or greater than that of iron at the peak of the binding energy curve, then the nuclear particles will .
Depleted Uranium
Nas aplicações atuais, o urânio é usado em reatores nucleares e armas militares.
Suggested for: A quick . The pellets are subsequently inserted into thin tubes known as fuel rods, which are then grouped together .Name of the isotope: Uranium-238; U-238 Other names: Uran I Symbol: 238 U or 23892 U Mass number A: 238 (= number of nucleons) Atomic number Z: 92 (= number of protons) Neutrons N: 146 Isotopic mass: 238.The most commonly defined as the fission energy release per unit mass of fuel in megawatt-days per metric ton of heavy metal of uranium (MWd/tHM) or similar units. • Over time, the activity of their decay products (initially zero) will increase.Last week, Centrus Energy in Bethesda, Md.
Diferença entre urânio 235 e urano 238: urânio 235 vs urânio 238
If a massive nucleus like uranium-235 breaks apart (fissions), then there will be a net yield of energy because the sum of the masses of the fragments will be less than the mass of the uranium nucleus. U238, on the other hand, is fissionable but requires a higher energy neutron to split.Both U238 and U235 can be used to make nuclear weapons, but U235 is the preferred isotope because it is easier to separate and has a higher probability of undergoing fission. So in operation some of .6Mev can split an U-238, while any neutron can split U-235. So for every seven or eight fissions, you have . Artificial isotopes.Sua reatividade nuclear é a principal razão para aquecer o núcleo da Terra e levar a fenômenos como a deriva continental. Spectral Variation of Neutron Cross Sections: Fe and Na. U235 is fissile in that it can fission with a zero energy neutron. Uranium has the highest atomic weight of all naturally occurring . Uran-238 ( 238U nebo U-238) je nejběžnější izotop uranu v přírodě s relativním zastoupením kolem 99,27 %. Although the process requires a large amount of the much rarer uranium-235 to start, it can be a good way to get more use out of the available uranium ore . Uranium-235 is produced through uranium processing in a centrifuge, or through the Kovarex enrichment process. Los principales usos del U-235 incluyen las aplicaciones en armas nucleares y plantas de energía nuclear., 2005, Zheng et al. It is also known as a radioactive cascade., 1991, McManus et al.In a nuclear reactor, non-fissile isotopes capture a neutron breeding fissile isotopes. Atomic number of both is 92, while neutron numbers of U238 and U235 are 146 and 143, respectively. Uranium-235 makes up about 0. In fact, U238 alone cannot sustain the chain reactions needed for nuclear power; conventional reactors require the fuel to be about 3. Sua reatividade nuclear é o principal motivo para aquecer o núcleo da Terra e levar a fenômenos como a deriva continental.
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. En la tabla periódica, su número atómico es 92 y su símbolo es U., jump-started the first commercial domestic nuclear fuel production in the United States in 70 years by delivering the first load of high-assay, low .
Diferencia entre el uranio 235 y el uranio 238 / Química
La fisión de un átomo U-235 libera 202.

Consider a uranium fueled reactor, consisting of enriched U235 and U238. The analysis, Section 4, is organized in two parts.
¿Qué es el uranio?
But we can look at the probabilities for each step.Uranium recovered from reprocessing of spent nuclear fuel is contaminated with fission products (mainly ruthenium-106 and technetium-99), with artificial uranium isotopes (U-232 , U-233, U-236, and U-237), with transuranics (such as neptunium-237 and plutonium-239), and with the decay products of all these nuclides.Three neutrons at once, no.In summary, U235 is the preferred isotope for chain reactions because it is fissile, meaning it can easily split upon absorbing a neutron.
Uranium 238
But why is the presence of the U-238 detrimental for the chain reaction of .Medicina nuclear: El uranio se utiliza en medicina nuclear en la producción de radioisótopos, como el tecnecio-99m, que se emplean en la obtención de imágenes médicas, como las tomografías por emisión de positrones (PET) y las gammagrafías, para el diagnóstico de enfermedades y trastornos. Propulsión espacial: La fisión nuclear del .There are three natural isotopes of uranium — uranium-234 (U-234), uranium-235 (U-235) and uranium-238 (U-238). U238 is fissionable with high energy neutrons and is also fertile in that through absorption of a neutron and subsequent beta decay a fissile isotope is produced, specifically Pu239. Required technologies.Fuel burnup (also known as fuel utilization) measures how much energy is extracted from nuclear fuel and measures fuel depletion in nuclear engineering.47×10 9 years), and therefore its abundance is so high.Its specific activity is much higher ~0.El uranio es un componente fundamental para la producción del combustible nuclear empleado en reactores nucleares de todo el mundo. É o único isótopo natural e físsil que é encontrado na natureza em quantidades relevantes, além de ser um .Energy from the uranium atom.05079 (2) u ( atomic weight of Uranium-238) Nuclide mass: 238.7 percent to 5 percent, the facility would produce 85 kg of enriched uranium and 915 kg of depleted uranium.Fissionable material consists of isotopes that are capable of undergoing nuclear fission after capturing either fast neutron (high energy neutron – let say >1 MeV) or thermal neutron (low energy neutron – let say 0. School science labs may keep small . This powder is then pressed to form small fuel pellets and heated to make a hard ceramic material.Therefore, an unstable nucleus continuously decays to achieve stability and so a lesser number of neutrons are stable and more radioactive in nature. The enriched uranium is transported to a fuel fabrication plant where it is converted to uranium dioxide powder. Nuclear Fuel Facts: Uranium. jednotky SI a STP (25 °C, 100 kPa). 238 U belongs to primordial nuclides because its half . Capture cross sections much higher in thermal range Significant . U-238 cannot easily absorb low-energy . U-238 is the most common one, accounting for around 99 per cent of natural uranium found on earth.
Enriched uranium
With respect to the values published in reference [2], our proposed values .The half-life of uranium 238 is of 4.For example, if an enrichment facility processes 1,000 kilograms (kg) of natural uranium to raise the U 235 concentration from 0. So, the easier the fuel is to split, the better.

The US Nuclear Regulatory Commission was aware of the existence of these trace contaminants in DU and determined them to be safe. counterweights).Uranium 235 vs Uranium 238. Os dois isótopos comuns de urânio são .
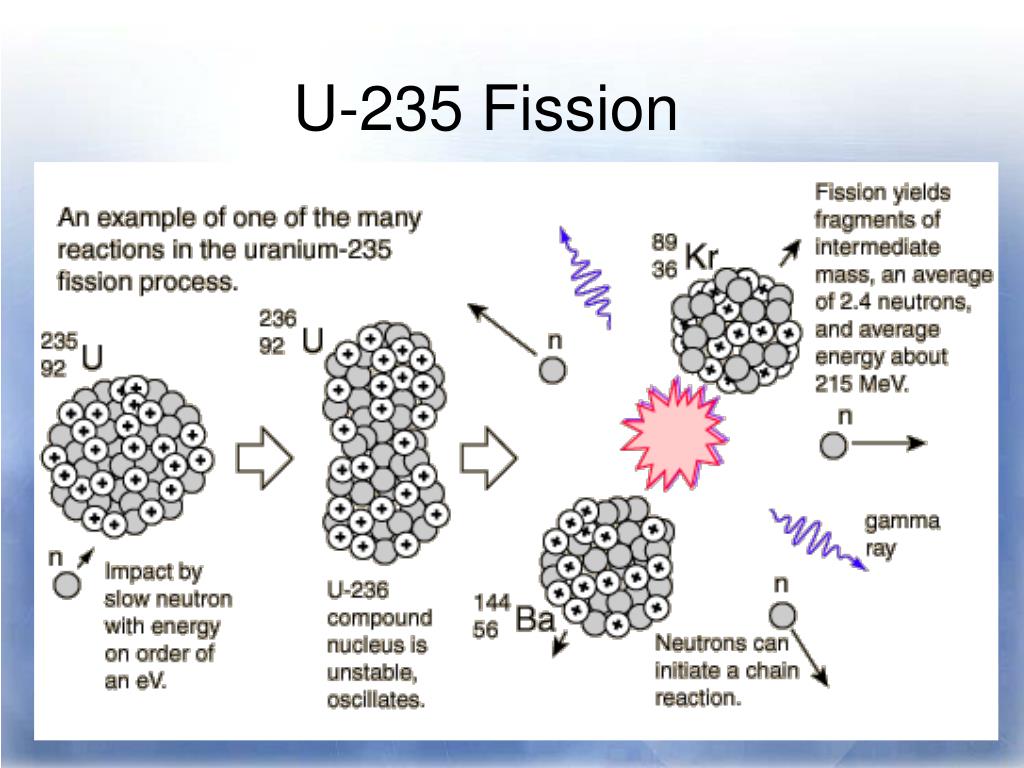
Each of these modes of decay leads to the formation of a new nucleus with a more stable n:p ratio. Fissile material consists of . Vargas/OIEA) El uranio es un elemento químico radiactivo que se encuentra presente en la naturaleza.
Why is U235 used for chain reaction but not U238
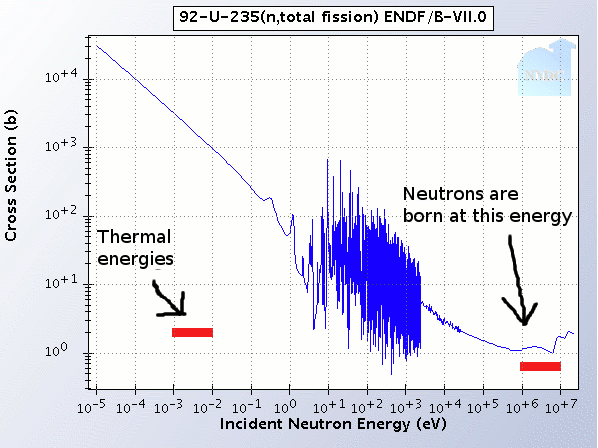
Urânio-235 é um isótopo de urânio que é responsável por cerca de 0.0003273 u (calculated nuclear mass without electrons) Mass excess: . The amount of U 235 in the bulk of the material decreases, or is depleted, to a concentration of 0. It is assigned the chemical symbol U. While both isotopes were at the time of Earth formation equally abundant, natural uranium today consists today of 99. Using these cross sections for thermal neutrons with energy 25 milli-eV, the total cross section for (n, fission) is about 460 barns, while the cross section for (n, gamma) (that is, capture to U-236) is about 60 barns.
Why Isotope of U235 is preferred as nuclear fuel over U238
Uranium 238 is a fissionable isotope but is not a fissile isotope.• B ti it it i hl 50% UBy activity, it is roughly 50% U-238 d 50% U238 and 50% U-234. While examining the sustainability of nu clear power, the use of thorium was given due considera tion from the early years of the nuclear power generation. U-235 has the ability to absorb thermal (low-energy) neutrons and fission, producing more neutrons. The radiation hazard to employees . Je však štěpitelný rychlými neutrony a je množivý, což .Plutonium-239 undergoes alpha decay to U-235, but that process has a half-life of 24,110 years., 2013, Klinkhammer and Palmer, 1991, Lovley et al. A centrifuge processing uranium ore will produce roughly 1 unit of uranium-235 for every 142 units of uranium . Uranium 238, which alone constitutes 99. In contrast, natural tho rium (Th232) is only a fertile material with no fissile content in it. Los dos isótopos comunes del .72 per cent of U . When the nucleus of a U-235 atom captures a moving neutron it splits in two (fissions) and releases some energy in the form of heat, also two or three additional neutrons are thrown off. The typical radioisotope does not decay directly to a stable state, but rather it decays to another radioisotope.Nuclear power plants rely on fission to operate. Por lo tanto, el U-235 es fisionable.5 Table 18: Changes ( in % )on the averaged values due to the adjustment f\ In spite of this, me ssrf prediction is improved, as revealed by a comparison of me Xr bcrore and arter adjustment. Diferente do isótopo predominante, o urânio-238, ele é físsil, ou seja, ele pode sustentar uma reação em cadeia de fissão nuclear. U-234 Th-230 (75,400 a) Although U-234 represents almost half the activity of pure uranium, the ingrowth of the U-234 daughters is relatively unimportant because: Purified Uranium – Daughter . 234 U is converted to 235 U more easily and therefore at a greater rate than uranium-238 is to plutonium-239 (via neptunium-239), because 238 U has a much smaller neutron-capture cross section of just 2. Una cadena de fisión natural terminará con Thorium-231, que es un elemento estable.The atomic bombs used on Hiroshima and Nagasaki were fission weapons. Os dois isótopos comuns do urânio são o U-235 e o U-238. Na rozdíl od uranu-235 je neštěpitelný, což znamená, že není schopen sám o sobě udržet řetězovou reakci.U235 and U238 are two member of Uranium isotope.3% uranium 238 and only 0. Esses isótopos mostram química semelhante, mas diferem nas propriedades físicas e .5 billion years, while uranium 235 has a half-life of ‘only’ 700 million years.
Fuel Burnup
kovarex-enrichment-process. Uranium 238 has the longest half-life (4. Typical fissionable materials: 238U, 240Pu, but also 235U, 233U, 239Pu, 241Pu.3 wt% U238 is fertile. Most nuclear reactors use fuels containing U-235, however, natural uranium typically contains only 0.
- Über Pc Anrufen Auf Handy _ Übers Internet telefonieren: Die besten Anbieter 2024
- Überwinterung Bei Tieren , Homoiotherm und poikilotherm Gleichwarm und wechselwarm
- U Bahn Karte Barcelona _ Barcelona: Tagestickets für Metro & Bus
- Types Of Cargo Transported By Air
- Two Letter Names For Boys | 100+ Three Letter Boy Names (Includes meanings and origins)
- Two Dimensional Array Examples
- Über The Air Update Erfahrungen
- Übermäßige Speichelproduktion _ Zu viel Speichel
- U2 Bahnhof München Karte _ U-Bahnhof Giesing (Bahnhof) (U2, U7, U8)
- Typische Brasilianische Gerichte