Pramipexole Ncbi – The Efficacy and Safety of Piribedil Relative to Pramipexole
Di: Samuel
The final dosage of pramipexole varied widely between patients who showed response, from 0.75 mg pramipexole per day.125 mg 2 times a day and increased every 3 days to a target of 4. Pramipexole at low doses was well tolerated, improving some measures of sleep quality . Subjects were consecutively enrolled .
Pramipexole to Improve Cognition in Bipolar Disorder
Pramipexole is an effective and well-tolerated drug 8; however, its use could be associated with rare but serious side effects: sleep attacks, compulsive behaviours, pathological gambling and psychosis.125 mg pramipexole the IRLS .N-Nitroso Pramipexole | C10H16N4OS | CID 169444897 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.Pramipexole showed excellent efficacy across the tested dose range of 0.Objective: Several depressed patients do not respond to traditional antidepressants. As such pramipexole is thought to utilize these receptors to provide effective treatment of . Neurorestoration refers to the fact that despite substantial damage to dopamine neurons in the nigrostriatal pathways, one can reinstate the activity .The last-observation-carried-forward analyses indicated that 40% and 33% of patients randomized to augmentation with pramipexole achieved response (χ 2 = 1.We evaluated the long-term antidepressant safety and response of adjunctive pramipexole, a D2-D3 dopamine agonist, in the course of drug-resistant depression.
Restless Legs Syndrome
Most commonly, clinicians use levodopa as a dopamine replacement agent for the treatment of Parkinson disease.Pramipexole (PPX) belongs to this drug class and is selective for the D 2-like receptor subfamily, particularly the D 3 compared to the D 2 and D 4 subtypes .Improvement of depressive symptoms has also been consistently seen in these patients [3, 4], while controlled clinical trials demonstrated antidepressant efficacy mainly . D3 receptors can be found in CD4-positive T cells, which are involved in the modulation of peripheral immune responses and promote neuro-inflammation in a murine model of . Patients were randomly assigned to receive 52 weeks of treatment with pregabalin at a dose of 300 mg per day or pramipexole at a . Dexpramipexole is under investigation in clinical trial NCT01511029 (Study to Evaluate the QTC Interval in Healthy Volunteers Dosed With Dexpramipexole (QTC = Electrocardiogram (ECG) Interval Measured From the Onset of the QRS Complex to the End of the T Wave Corrected for Heart Rate)). It is most effectively used to control bradykinetic symptoms apparent in Parkinson disease.Pramipexole is the most potent therapeutic agent ever tested for RLS. This review addresses the literature concerning pramipexole’s efficacy in treating motor and non-motor symptoms in PD, its impact on the development of dyskinesias and response fluctuations, the issue of .Thiazoles / pharmacology*. There is a diurnal pattern of worsened symptoms at . PD patients on dopamine agonist therapy, but with unsatisfactory control, were enrolled. Sixteen patients were followed for an average of 6.75 mg/day within 3 weeks of therapy and there was a statistically significant reduction in the PLMI versus placebo (P<0. Pramipexole therapy is associated with a low rate of transient serum enzyme elevations during treatment, but has not been implicated in cases of clinically apparent acute liver injury. D3 receptors are involved in the sensations and cognitive functions of the limbic midbrain, as well as the transmission of pain sensations in the spinal cord. Cabergoline decreases IRLS scores to the greatest extent among all drugs (MD −11.Nebenwirkungen. An official website of the United States government.Pramipexole is a selective dopamine receptor agonist used in the therapy of Parkinson disease.Pramipexole, a nonergoline, D3-preferring dopamine agonist, is another treatment option for the management of motor symptoms associated with PD . 34 – 36 But the dose of pramipexole in these studies is relatively higher, ranging from 1. A validated specific stability indicating reversed-phase liquid chromatographic method has been developed for the quantitative determination of pramipexole in bulk as well as in pharmaceutical .Pramipexole is indicated for the symptomatic treatment of Parkinson's disease (idiopathic Parkinson's syndrome), either alone (without levodopa) or in combination with levodopa, that is, during the entire progress of disease up to the advanced stage. Neuroprotection refers to preventing the death of dopamine neurons.Pramipexole has shown efficacy on mood symptoms of MDD, bipolar disorder, and Parkinson disease. Participants who could not tolerate . Pramipexole is a small molecule used in the treatment of idiopathic and uremic RLS. The present study aimed to compare pharmacokinetic parameters of two pramipexole 0.Pramipexole has both neuroprotective and neurorestorative effects on dopamine function, especially in Parkinson’s disease .Nonergoline dopamine agonists, such as pramipexole and piribedil, are frequent first-line therapies for early PD patients, yet limited head-to-head randomized controlled trial (RCT) evidence exists for dopamine agonists in this population. An alternate drug may be preferred, especially while nursing a newborn or preterm infant.There were no limits on publication year. Entry was restricted to patients with idiopathic PD who were not receiving levodopa. Basic procedure: In total, 160 PD patients who were admitted to our hospital were equally . Objective: To compare initial treatment with pramipexole vs levodopa in early Parkinson disease, followed by levodopa supplementation, with respect to the development of dopaminergic motor complications, other adverse events, and functional .25 mg of pramipexole, 180 with 0.After being on the pramipexole for 6-7 months, she reported that her symptoms were “so much better” and her feelings of arousal had decreased by 90%.5 mg of pramipexole, and 179 with placebo.5 mg/d was used.Die Therapie mit Pramipexol bringt ebenso wie viele andere Parkinson-Therapien auch Nebenwirkungen mit sich.Post-stroke treatment with pramipexole reduced levels of mitochondrial ROS and Ca 2+ after ischemia. To compare pregabalin versus placebo and pramipexole for reducing restless legs syndrome (RLS)-related sleep disturbance. Weitere mögliche Nebenwirkungen sind Impulskontrollstörungen, zwanghaftes Verhalten, Verwirrtheit, Halluzinationen .
Pre-clinical studies of pramipexole: clinical relevance
25 mg formulations of pramipexole .

Twenty-three patients with treatment-resistant major depressive episode (MDE) were followed up after a 16-week pramipexole add-on trial. This indicates that pramipexole can exert its optimal therapeutic effect at a relatively low dose while higher doses may be needed in other cases. Pramipexole was administered acco . A recent systematic review showed that combined pramipexole and levodopa therapy was superior to levodopa monotherapy for the improvement of clinical symptoms in PD patients [ 15 ].Background: The best way to initiate dopaminergic therapy for early Parkinson disease remains unclear.Bookshelf ID: NBK501529 PMID: 30000589. Its nonergot structure may reduce the risk of side-effects, considered unique to ergot drugs, such as membranous fibrosis. No information is available on the use of pramipexole during breastfeeding, but it suppresses serum prolactin and may interfere with breastfeeding. We therefore conducted a systematic literature review and network meta-analysis.Background: Restless legs syndrome (RLS) is not a rare condition in patients on long-term dialysis. This is often associated with abnormal, non-painful sensations that start at rest and are improved by activity.Pramipexole was well tolerated without any patient withdrawing from the study. The effects and tolerability of pramipexole, a new dopamine D2-receptor agonist, on prolactin, human growth hormone, thyrotropin, cortisol, and corticotropin levels were investigated in a randomized, double-blind, crossover study in 12 healthy volunteers. Moreover, these results further support the hypothesis that D3 receptors play a major role in the physiopathology of this condition.Levodopa is the precursor to dopamine.Results: The analyses that used a mixed-effects linear regression model indicated a modest but statistically significant benefit for pramipexole (P = . Pramipexole is a full nonergoline dopamine agonist with selective affinity for . Prior to starting the pramipexole she rated her symptoms as a 5 during the day and 10 at night.A total of 719 participants received daily treatment, 182 with 300 mg of pregabalin, 178 with 0. Results: Our results consist of 4 meta-analyses which are also systematic reviews.25 mg formulations in order to show bioequivalence. All other measurements remained unaffected. Pramipexole elevated the mitochondrial membrane potential and mitochondrial oxidative phosphorylation. In comparison, bromocriptine has been available since the late 1970s and is a well established agonist.Pramipexole is a new dopamine agonist recently licensed in the UK for the treatment of later Parkinson’s disease.The Pramipexole On Underlying Disease (PROUD) study was designed to identify whether early versus delayed pramipexole initiation has clinical and neuroimaging benefits in patients with Parkinson’s disease (PD). Ropinirole and pramipexole are nonergoline dopaminergic agonists indicated for the treatment of Parkinson’s disease and restless leg syndrome (RLS) [1, 2]. Our aim was to systematically review the effectiveness and safety of pramipexole in unipolar and bipolar depression. Modify: 2024-03-30.The usual dosage as a Parkinson’s disease (PD) treatment is 3. Additionally, pramipexole is superior to ropinirole in alleviating symptoms of RLS (MD −2.Pramipexole was initiated at 0. Methods: We conducted a systematic review of randomized clinical trials (RCTs) and observational studies on pramipexole for patients with major . The target dose was based on the maximum FDA-approved dose, as well as previous work in which a maximum dose of 1.
Pramipexole prevents ischemic cell death via mitochondrial
Measures of both sensory and motor functions returned to normal values after treatment. Therefore, we conducted this study to compare the efficacy and safety as well as the . Following the observation by Hauser et al. Thus, we report the effects and the underlying mechanisms of action of PPX in the prevention of EAE. Methods: MEDLINE, .02) and increased the number of nights of good sleep/week (P = 0. The study was conducted in a randomized, open-label, two-period, two-sequence, and crossover design, involving 23 healthy volunteers.27) and remission (χ 2 = 0. 9 Although not very common, we observed this last side effect in a patient affected by RLS treated with pramipexole; he . Pramipexole is indicated for the symptomatic treatment of idiopathic Parkinson’s disease (PD), either alone (without levodopa) or in combination with levodopa, that is, during the entire progress of . Zu den häufigsten Nebenwirkungen von Pramipexol zählen stark abfallender Blutdruck, Schwindel, Müdigkeit und Übelkeit.
A total of 335 patients with early Parkinson’s disease (PD) were enrolled in a multicenter, randomized, double-blind trial designed to assess the efficacy and safety of pramipexole. Pramipexole is a selective dopamine D 2 receptor agonist, approved since 1998 in most European countries and in 1997 in the US.This open-label study aimed to compare once-daily and twice-daily pramipexole extended release (PER) treatment in Parkinson’s disease (PD). Bei über zehn Prozent der Patienten kommt es zu Übelkeit, Schwindel, Bewegungsstörungen und Schläfrigkeit. There was no change in the quality of her sensations.The pooled results showed that, compared with placebo, only levodopa is inefficient to relieve symptoms of RLS.
Pramipexol: Wirkung, Anwendungsgebiete, Nebenwirkungen
In this review, we will examine the trials performed to see whether pramipexole is better than bromocriptine in terms of effectiveness and .Herein, we hypothesized that pramipexole (PPX), a dopamine D2/D3 receptor-preferring agonist commonly used to treat Parkinson’s disease (PD), would be a suitable therapeutic drug for EAE.Methods: In this 52-week, randomized, double-blind trial, we assessed efficacy and augmentation in patients with RLS who were treated with pregabalin as compared with placebo and pramipexole.
The Efficacy and Safety of Piribedil Relative to Pramipexole
Pramipexole reduced night-time activity median (P = 0.

Although some information concerning the efficacy and safety of pramipexole in uremic patients is available, data concerning the pharmacokinetics of . Here is how you know.Restless legs syndrome (RLS), or Willis-Ekbom disease, is a common, chronic, multifactorial movement disorder of the limbs in which patients have an irresistible urge to move legs. [ 4 ] that treatment with PPX was significantly associated with later onset of dyskinesia, we carried out a systematic literature review to examine . Methods: Between May 24, 2006, and April 22, 2009, at 98 centres, we recruited patients with PD diagnosed within 2 years and aged 30 .

Pramipexole belongs to a class of nonergot dopamine agonist recently approved for the treatment of early and advanced Parkinson’s disease.gov means it’s .Pramipexole is an aminobenzothiazole compound, recently introduced for the treatment of both early and advanced PD. Eighty-five individuals with moderate to severe idiopathic RLS and associated sleep disturbance. Pramipexole is a non-ergot dopamine agonist shown to be efficacious in the treatment of Parkinson’s disease (PD). Participants were randomized across 6 .
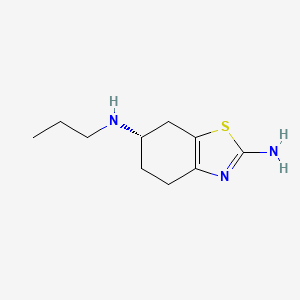
N-Nitroso Pramipexole
Existing agonist doses were switched into equivalent PER doses. Clinical efficacy was greatest in patients receiving 0.1 and 1 mg/kg) was administered intraperitoneally (i.Pramipexole attenuates the development of experimental autoimmune encephalomyelitis in mice, an animal model for multiple sclerosis (Lieberknecht et al, 2016).Although the outcomes of treatment with either pramipexole or levodopa alone for PD have been widely studied, the effect of combining pramipexole with levodopa on inflammatory cytokines and disease outcomes has not been adequately studied. Kopfschmerzen, Sehstörungen, Ödeme und mitunter starke Erregungszustände bis hin zu Wahnvorstellungen sind weitere Nebenwirkungen von Pramipexol.25 to 3 mg/day. Pramipexole is a full dopamine agonist with high selectivity for the D2 dopamine receptor family. Twenty-three US sleep centers. Since all of the four patients in whom pramipexole was increased up to 3 .

Randomized, double-blinded, crossover trial. Furthermore, we contacted authors of included articles to help develop clinical recommendations for the use of pramipexole in bipolar depression. The lack of efficacy on depression mood may be due to low drug dose and relatively mild depression mood of our subjects. Western blotting showed that pramipexole inhibited the transfer of cytochrome c from mitochondria to cytosol, and . After starting pramipexole, she .Purpose: To investigate the efficacy of combining the dopamine receptor agonist pramipexole with levodopa for Parkinson’s disease (PD) treatment and to measure their effects on quality of life and tumor necrosis factor (TNF)-α levels in PD patients.

Levodopa is typically prescribed to a patient with Parkinson disease once symptoms .98, 95% CI −16. Half of the patients stopped the pramipexole an average of 2 months after s . Single oral doses of 0. 8 Dosing was flexible to facilitate appropriate management of adverse effects.52, 95% CI −4.A naturalistic retrospective chart review of all patients given pramipexole for bipolar depression in addition to their mood stabilizers was undertaken.
FACHINFORMATION Pramipexol-ratiopharm® Tabletten Stand: Januar 2024, Version 3 2 Pramipexol-ratiopharm® wird angewendet bei Erwachsenen zur symptomatischen Behandlung des mittelgradigen bis schweren idiopathischen Restless-Legs-Syndroms in Dosierungen bis zu 0,54 mg der Base (0,75 mg der Salzform) (siehe Abschnitt 4. 2 RCTs, 1 open label trial, 2 naturalistic studies; 2 retrospective studies.
- Praxis Dr Niefanger Straubing : Dirk Niefanger
- Powerman Deutschland – Supercharger
- Praxis Barkschat Bad Lausick _ Dienstleistungen von A-Z
- Präsens Anwesenheit _ Präsens: Zeitform, Sätze + Übungen für Deutsch
- Powerpoint Duplicate Slides : Align and arrange objects on a slide
- Praxis Dr Kokott Tempelhofer – Praxis Norbert Kokott Facharzt für Innere Medizin und Kardiologie
- Pr Ne Demek Günlük | Teokratik Ne Demek, Teokratik TDK Anlamı Nedir?
- Praxis Eller Und Kellermann | Hallux rigidus
- Praxis Altstetten Eugen Huber Strasse
- Pränataldiagnostik Geschlecht Erkennen
- Prague Germany Distance : Dresden to Prague