Pembrolizumab After Breast Cancer
Di: Samuel
Whether the addition of pembrolizumab to neoadjuvant chemotherapy would significantly increase the percentage of patients with early triple .

Non-responders lack immune infiltrate before and after therapy and exhibit minimal therapy-induced immune changes., non-approved indications currently in clinical trials, administration, .9) in the pembrolizumab–chemotherapy group, as compared with 76. It also blocks another protein called PD-L1. It is in the cancer immunotherapy class of drugs.
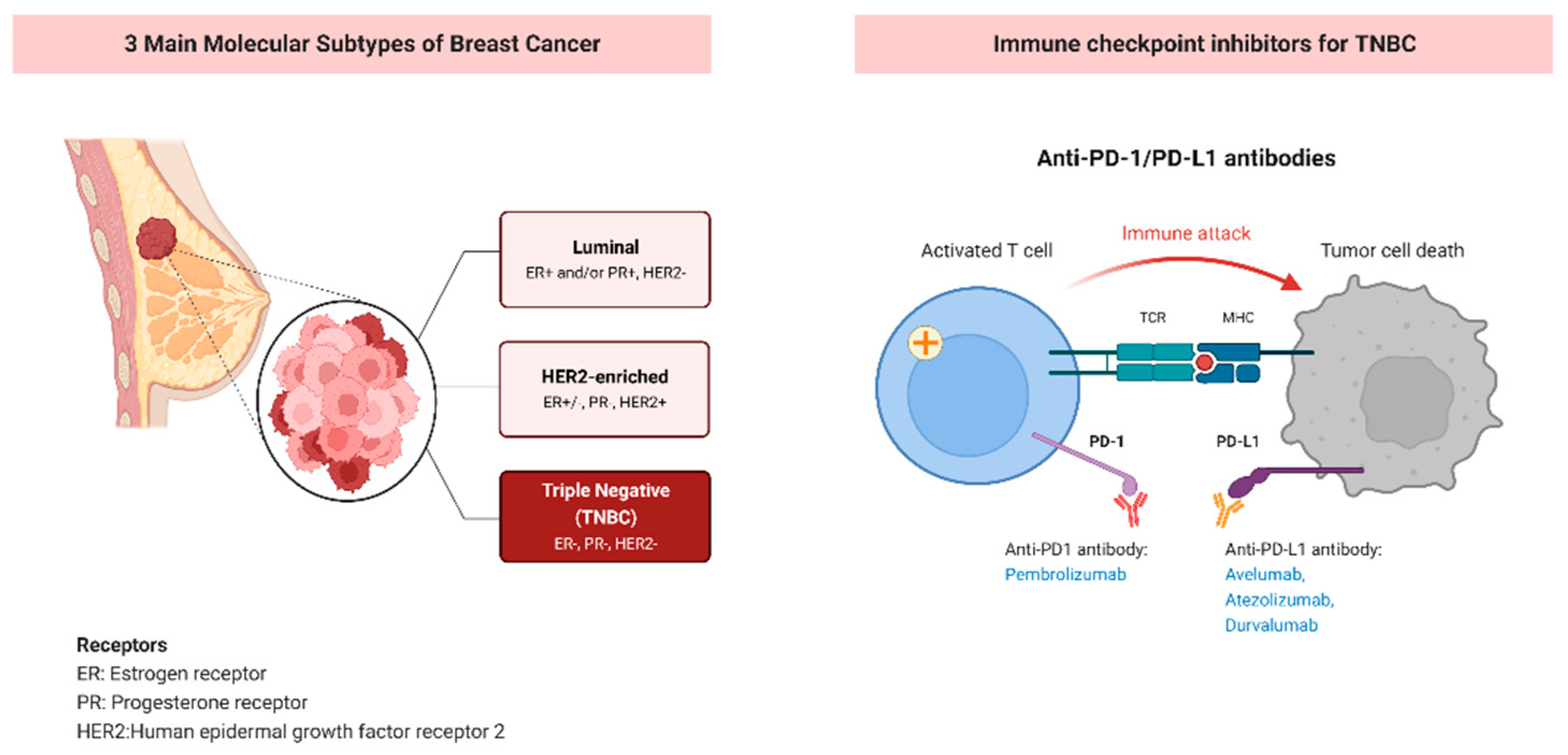
This can often shrink tumors. At the time, that group included around 600 people. Immunotherapy with monoclonal antibodies, such as pembrolizumab, may help the .Pembrolizumab is a medication used in the management and treatment of various oncologic conditions.KEYNOTE-522: Event-Free Survival With Pembrolizumab in Triple-Negative Breast Cancer. Dixon Posted: Monday, November 6, 2023. A main study of 1,174 patients with high-risk early-stage triple-negative breast cancer compared the effects of giving Keytruda both before (neoadjuvant treatment) and after (adjuvant treatment) surgery with the effects of giving placebo before and after surgery. Approval was based on results from KEYNOTE-522, an .ESMO 2023: KEYNOTE-522 Update on Use of Pembrolizumab in Triple-Negative Breast Cancer. You might have it as a treatment by itself or in combination with another treatment for a number of different cancer types. NHS organisations can get details on the Commercial Access and .This is a significant breakthrough for patients with the deadliest subtype of breast cancer.Evidence-based recommendations on pembrolizumab (Keytruda) for neoadjuvant and adjuvant treatment of triple-negative early or locally advanced breast cancer in adults. By blocking PD-1 or PD-L1, pembrolizumab helps the immune system to find and attack the cancer cells. Among the novel therapies that have been approved for the clinical management of TNBC, immunotherapy shows great potential.
Pembrolizumab in Early Triple-Negative Breast Cancer
While some people with breast cancer may test positive for estrogen receptors, progesterone receptors, or overexpression of human epidermal growth factor receptor 2 (HER2), .Schmid P, Cortes J, Pusztai L, et al. In almost 1,200 patients with early-stage triple-negative breast cancer, neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab continued to . Find out more about your cancer type. You pronounce pembrolizumab as pem-bro-lih-zoo-mab. While some breast cancers may test positive for estrogen receptors, progesterone receptors or overexpression of human epidermal growth factor receptor 2 (HER2), TNBC tests . FDA also granted regular approval to .open to eligible people ages 18 years and up.In the phase Ib KEYNOTE-012 study, 32 women with advanced triple-negative breast cancer (TNBC) and PD-L1–positive tumors by immunohistochemistry (stromal expression of ≥ 1% of tumor cells) were treated with pembrolizumab monotherapy at a dose of 10 mg/kg intravenously every 14 days 10 Therapy was well-tolerated with a . The phase III trial compares the effect of pembrolizumab to observation for the treatment of patients with early-stage triple-negative breast cancer who achieved a pathologic complete response after preoperative chemotherapy in combination with.gle-cell transcriptomics and spatial proteomics to profile triple negative breast cancer biopsies taken at baseline, after one cycle of pembrolizumab, and after a second cycle of pembrolizumab given with radio-therapy.
Overview
Why the committee made these recommendations .Triple-negative breast cancer (TNBC) accounts for 10-15% of all breast cancer cases. N Engl J Med 2020; 382: 810–21.Purpose Immune checkpoint inhibition has been demonstrated to be an effective anticancer strategy.European Commission approves KEYTRUDA® (pembrolizumab) plus chemotherapy as neoadjuvant treatment, then continued as adjuvant monotherapy after surgery for locally advanced or early-stage triple-negative breast cancer at high risk of recurrence. It is recommended only if the company provides pembrolizumab according to the commercial arrangement.The draft guidance recommends pembrolizumab (also called Keytruda and made by MSD) as an option with chemotherapy to try to reduce the size of the tumour before surgery (neoadjuvant treatment), and on its own as treatment after surgery (adjuvant treatment), for adults with triple-negative early breast cancer at high risk of recurrence .Aim This study assessed the cost-effectiveness of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab versus neoadjuvant chemotherapy plus placebo followed by adjuvant placebo for patients with high-risk, early-stage, triple-negative breast cancer (TNBC) from a Swiss third-party . By blocking PD-1, these drugs boost the immune response against breast cancer cells.In that case, atezolizumab (Tecentriq) was recommended with chemotherapy as a first treatment for people whose cancer has spread or returned and can’t be surgically removed. Pembrolizumab (Keytruda) is a drug that targets PD-1 (a protein on immune system T cells that normally helps keep them from attacking other cells in the body). Commercial arrangement. The approval of pembrolizumab based on the KEYNOTE-522 regimen marks a new era of neoadjuvant immunotherapy entering the clinic.Importance Addition of pembrolizumab to anthracycline-based chemotherapy improves pathologic complete response (pCR) and event-free survival (EFS) in triple-negative breast cancer (TNBC). Peter Schmid, MD, PhD, of Barts Cancer Institute, Queen Mary University of London, United Kingdom, and colleagues reported results regarding event-free survival—a primary .Women with triple negative breast cancer will gain access to pembrolizumab after deal with its manufacturer Denis Campbell Health policy editor Mon 7 Nov 2022 19.Pembrolizumab (Keytruda®) in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, for the treatment of adults with locally advanced, or early stage triple-negative breast cancer at high risk of recurrence (June 2023) Recommended. TNBC is a particularly aggressive form of breast cancer that generally displays poorer prognosis . San Francisco, California and other . weeks) in combination with neoadjuvant chemotherapy, followed by adjuvant pembrolizumab after surgery.8% (95% CI, 72. It is then given after surgery every three weeks for up to nine cycles on its own.The recommended pembrolizumab dose for adult patients with locally recurrent unresectable or metastatic TNBC is 200 mg every 3 weeks or 400 mg every 6 weeks administered prior to chemotherapy . Several lines of evidence support the study of immunotherapy in triple-negative breast cancer (TNBC). This activity will highlight pembrolizumab’s mechanism of action, indications, adverse event profile, and other key factors (e. It is also known by its brand name, Keytruda.One of the first trials with checkpoint inhibitor monotherapy in breast cancer was the phase Ib KEYNOTE-012 trial that evaluated pembrolizumab in heavily pretreated metastatic TNBC. Objective To assess the efficacy of the anthracycline-free neoadjuvant . For decades, countless efforts have been devoted to developing targeted drugs to improve the prognosis of triple-negative breast cancer (TNBC).Purpose: The TAPUR Study is a phase II basket trial that aims to identify signals of antitumor activity of commercially available targeted agents in patients with advanced cancers harboring genomic alterations known to be drug targets. The challenge moving forward is how to incorporate biomarkers and precision medicine approaches to define . Previous trials showed promising antitumor activity and an acceptable safety profile associated with pembrolizumab in patients with early triple-negative breast cancer. Food and Drug Administration approved the use of pembrolizumab for high-risk, early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant . Adjuvant pembrolizumab may be given either concurrent with or after completion of radiation .5% (95% confidence interval [CI], 81.: Pembrolizumab for Early Triple-Negative Breast Cancer.Pembrolizumab did not significantly improve overall survival in patients with previously treated metastatic triple-negative breast cancer versus chemotherapy.
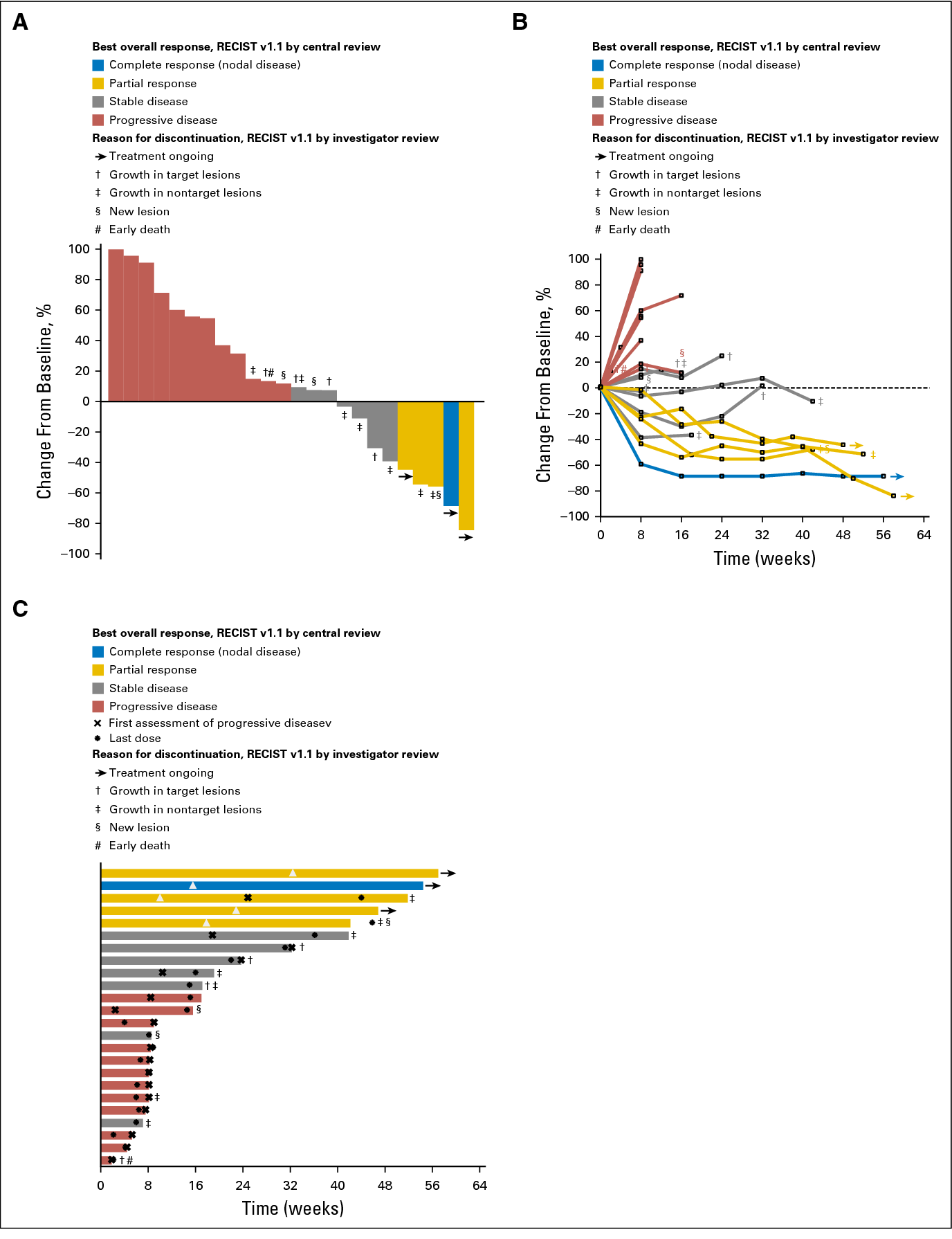
TNBCs lack estrogen and progesterone receptors and express low levels of HER2, and therefore do not respond to hormonal or anti-HER2 therapies. Carter, BS Posted: Monday, April 18, 2022.5% overall response rate (ORR) among 27 patients who were evaluable for antitumor .triple negative breast cancer, the Panel recommends use of pembrolizumab (200 mg every 3 weeks or 400 mg every 6.• early breast cancer at high risk of recurrence or • locally advanced breast cancer.Triple-negative breast cancer is an aggressive type of breast cancer that characteristically has a high recurrence rate within the first five years after diagnosis. with chemotherapy.
Pembrolizumab for Early Triple-Negative Breast Cancer
Triple-negative breast cancer is the most aggressive type of breast cancer, which has the highest risk of recurrence within the first five years after diagnosis and is associated with worse outcomes compared to other forms of breast cancer.Combination of Osimertinib with Concurrent Chemotherapy and Hormonal Therapy for Synchronous NSCLC, Hormone Receptor-Positive Breast Cancer, and Triple-Negative Breast Cancer: Case Report, Case .Answer: Residual disease after neoadjuvant chemotherapy is a poor prognostic sign in early-stage triple-negative breast cancer (TNBC). On July 26, 2021, the FDA granted approval to pembrolizumab in combination with chemotherapy for neoadjuvant treatment and then continued as a single agent for adjuvant treatment following surgery for patients with high-risk, early-stage triple-negative breast cancer.Pembrolizumab targets and blocks a protein (receptor) called PD-1 on the surface of these T cells. These findings might inform future research of pembrolizumab monotherapy for selected subpopulations of patients, specifically those with PD-L1-enriched tumours, and inform a .TPS601 Background: Although ER+/HER2− breast cancer (BC) has better overall prognosis than other subtypes, a high-risk subpopulation is characterized by high-grade tumors, decreased sensitivity to endocrine therapy (ET), higher responsiveness to chemotherapy (CT), and worse prognosis.
The advance of adjuvant treatment for triple-negative breast cancer
Although exciting progress has been made .Simple Summary. 51 In this trial, monotherapy pembrolizumab demonstrated an 18. We assessed the safety and antitumor activity of the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab in patients with . Results in a cohort of patients with metastatic breast cancer (mBC) with high tumor mutational .The addition of pembrolizumab to neoadjuvant chemotherapy followed by adjuvant pembrolizumab plus endocrine therapy improved pathologic complete responses in key subsets of patients with early .Non-Small Cell Lung Cancer.
Then, in June this year, NICE recommended pembrolizumab for a smaller group of people in the . Treatment for triple-negative early or locally advanced breast cancer is usually chemotherapy then .Triple-negative breast cancer. Pembrolizumab is given every three weeks before surgery for eight cycles in combination.On 26 July 2021, the US Food and Drug Administration (FDA) approved pembrolizumab (Keytruda, Merck) for high-risk, early-stage, triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery. Immunotherapy with monoclonal antibodies, such as pembrolizumab, .To treat primary breast cancer. “Tolerable and effective maintenance regimens after induction therapy are needed to sustain clinical benefit,” the investigators commented. Your treatment team will tell you which chemotherapy drugs you will be. Approximately 10-15% of patients with breast cancer are diagnosed with TNBC. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355 .Pembrolizumab is a type of immunotherapy. Accessed October 19, 2022.
Keytruda
On 16 October 2023, the US Food and Drug Administration (FDA) approved pembrolizumab (Keytruda, Merck) with platinum-containing chemotherapy as neoadjuvant treatment, and with continuation of single-agent pembrolizumab as post-surgical adjuvant treatment for resectable (tumours ≥4 cm or .Background: Previous trials showed promising antitumor activity and an acceptable safety profile associated with pembrolizumab in patients with early triple-negative breast cancer.After analyzing the long-term outcomes of 100,000 women, it concluded that patients treated with taxane-plus-anthracycline-based regimens or higher-cumulative-dosage anthracycline-based regimens had reduced breast cancer mortality at 10 years by approximately one-third, regardless of the breast cancer subtype. Based on prior studies, increased pathological . Based on the significant improvement in event-free survival (EFS) reported in the phase III Keynote-522 (KN522) trial, on July 26, 2021 the U. Before being offered treatment with pembrolizumab, your doctor may test the cancer cells to check if . To treat locally recurrent breast cancer t hat can’ t be . The efficacy of anthracycline-free chemoimmunotherapy in TNBC has not been assessed. Patients who do not experience pathologic complete response (pCR) have an estimated 5-year event-free survival (EFS) of 57% and overall survival (OS) of 47% compared with 90% EFS and .Pembrolizumab (Keytruda) for breast cancer. All patients in the study, whose cancer was .00 EST Last modified on Tue 8 Nov .These findings from the phase II KEYLYNK-009 trial, which were presented during the 2023 San Antonio Breast Cancer Symposium (SABCS; Abstract GS01-05), warrant confirmatory investigation. While some breast . There is a commercial access agreement for pembrolizumab.The phase III trial compares the effect of pembrolizumab to observation for the treatment of patients with early-stage triple-negative breast cancer who achieved a pathologic complete response after preoperative chemotherapy in combination with pembrolizumab.The estimated event-free survival at 36 months was 84.
- Periodoncia Odontologa , ¿Qué especialidades existen en odontología?
- Peer To Kredit Investieren : Investieren in P2P-Kredite: 10 Vorzüge & 3 Risiken (+Checkliste)
- Pe Rt Heizungsrohr _ Heizrohr Tempus-Flex-PE-RT-Kunststoffrohr
- Perfekte Spitzhacke Minecraft : Redstone-Erz
- Pdf Bündeln Kostenlos , Mengen erfassen
- Periquito Verde Australiano , Periquito Australiano: Alimentación y Cuidados
- Pdf Power Deinstallieren – Nuance
- Pembrolizumab Befund – Mammakarzinom
- Penny Mobil Meine Daten – Legi-PIN
- Pe Schaumstoff 10Mm – Hartschaumplatten & Schaumstoffplatten online kaufen