Nmr Chart – NMR Spectroscopy- Definition, Principle, Steps, Parts, Uses
Di: Samuel
For example, if we were measuring the 1 H NMR spectrum of a sample using an instrument operating at 200 MHz, 1 δ would be 1 part per million of 200,000,000 Hz, or 200 Hz. The concepts implicit and fundamental to the operation of a modern NMR spectrometer, with generic illustrations where appropriate, will be described.Using NMR Chemical Impurities Tables. Assign 1H NMR spectra to molecule. Carbon-13 nuclei fall into a class known as spin ½ nuclei for reasons which do not really need to concern us at the introductory level this page is aimed at (UK A level and its equivalents). By remembering the positions of these regions, it’s often possible to tell at a glance what .
NMR Periodic Table
Example \(\PageIndex{2}\) Using the chemical formula and 1 H NMR spectrum, determine the structure of your unknown molecule. In general, protons follow the trend seen in the carbon to which they are attached.5 C ≡ C–H acetylenic 2-3 Ar–H aromatic 6-8.Numeraire (NMR) – Bullish divergence On the above 1-day chart price action has corrected almost 90% from $90 since May 2021.13C NMR Chemical Shift Table 140. The current CoinMarketCap ranking is #312, with a live market cap of $194,950,521 USD.9 C=C– H vinylic, conjugated 5. Aires-de-Sousa, Prediction of 1H NMR coupling constants with associative neural networks trained for chemical shifts J.
1H-NMR-Spektroskopie
Nuclear magnetic resonance spectroscopy
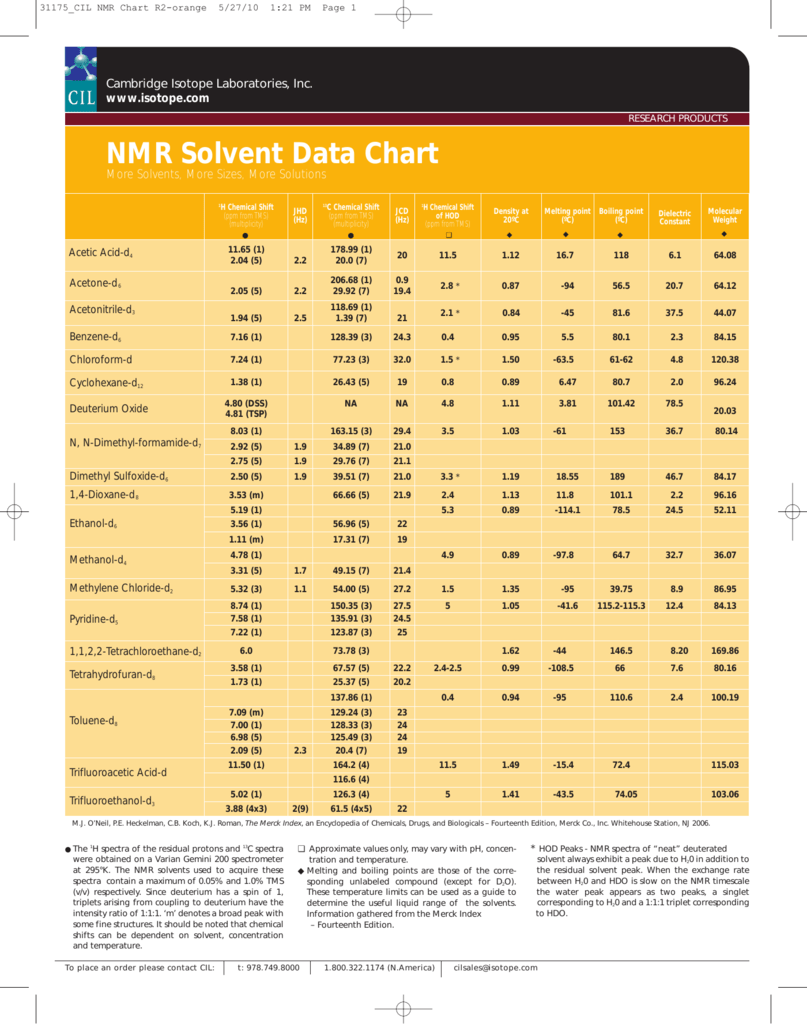
The two peaks around 130 must be the two carbons at either end of a carbon-carbon double bond. First, each spectrum consists of a set of peaks, each of which is a singlet, suggesting that no spin-spin coupling is taking place.org: Resurrecting and processing .NMR spectra were taken in a Bruker DPX-300 instrument (300.Table of Characteristic Proton NMR Shifts type of proton type of compound chemical shift range, ppm RCH 3 1˚ aliphatic 0. NMR Active Nuclei Accessible with a Low-Frequency NMR Probe. Use the chart below to look up the coupling values — J HD and J CD (J CF) distance between multiplet peaks in hertz (Hz) — and chemical shift delta values — ð H (Mult) b and ð c (Mult) b in parts per million (ppm) — of NMR solvents by name or CAS number. 2007, 47/(6), 2089-2097.5 MHz for 1Hand13C, respectively). It is less common than H-1 but is still regularly used in organic chemistry laboratories. 1H NMR basic structure assignment.9 C=C–H vinylic, conjugated 5.No two carbons are in exactly the same environment. Frequency at (300 MHz) Frequency at (400 MHz) Frequency at (500 MHz) Frequency at (600 MHz)
Table of Characteristic Proton NMR Shifts
5 Ar–C– H benzylic 2. In case of one . It is used in research for determining the content and purity of a sample as well as its molecular structure.5 C! C– H acetylenic 2–3 Ar– H aromatic 6–8.Table of 1 H NMR Frequencies Common in Organic Compounds. 1997, 62, 7512) by .2-3 C=C–CH 3 allylic 1.5 Ar–C–H benzylic 2.1 H NMR prediction was possible thanks to the tool of the FCT-Universidade NOVA de Lisboa developped by Yuri Binev and Joao Aires-de-Sousa.
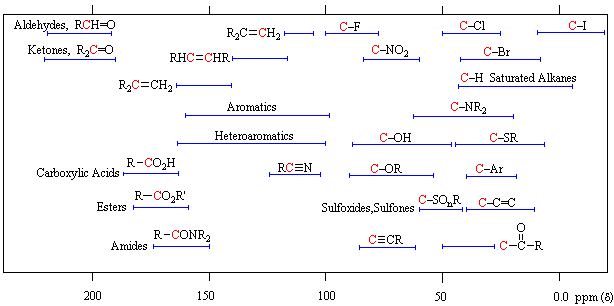
Most 1 H chemical shifts fall within the range 0 to 10 δ, which can be divided into the five regions shown in Table 13.0 130 110 215 200 180. The effect of this is that a C-13 nucleus can behave as a little magnet. In cases where a proton is influenced by more than one group, the effects are essentially cumulative, for example proton shift in CH3Cl is at approximately 3.This set of pages originates from Professor Hans Reich (UW-Madison) Structure Determination Using Spectroscopic Methods course (Chem 605).4 • Chemical Shifts in 1 H NMR Spectroscopy As mentioned previously, differences in chemical shifts are caused by the small local magnetic field of electrons surrounding different nuclei. This table does not include OH (or NH) protons.5 C=C–H vinylic 4. Use chemical shift tables or charts to . Zusätzlich wird eine Feinaufspaltung der Banden beobachtet, die wie-derum anzeigt, wie viele andere Wasserstoffkerne sich in der Umgebung der betreffenden Sorte Wasserstoffatome befinden.4: Tosylate—Another Good Leaving Group is shared under a CC BY-NC-SA 4.Nuclei that are less strongly shielded need a lower applied field for resonance so they absorb on the left of the NMR chart.Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field.5 C=C– H vinylic 4.C-13 NMR relies on the magnetic properties of the C-13 nuclei. Nuclei that are more strongly shielded by electrons require a higher applied field to bring them into resonance so they absorb on the right side of the NMR chart.9 R 2 CH 2 2˚ aliphatic 1. NMR Active Nuclei. The position and number of chemical shifts . Number of different Hs. It describes Nuclear Magnetic Resonance (NMR) in details relevant to Organic Chemistry. For the experiments in the last section of this paper, probe temperatures weremeasured with a calibrated Eurotherm 840/T digital thermometer, connected to a . Unless otherwise indicated, all were run at room temperature (24 ( 1 °C).Die NMR-Spektroskopie liefert dabei Banden, deren Lage Aufschluss über die chemische Umgebung der zugehörigen Wasserstoffatome gibt. This work supplements the compilation of NMR data published by Gottlieb, Kotlyar, and Nudelman (J.C-13 NMR spectroscopy is specific to the analysis of the carbons in a molecule. C-13 NMR can give the following information: . Unknown molecule 1 H NMR spectrum: The ratio of protons is 2:2:2:3. There are three things to make note of from this figure. This chart shows the frequancies of protons that are attached to carbons. Unlike 1 H-NMR signals, the area under a 13 C-NMR signal cannot be used to determine the number of carbons to which it corresponds.The 1H and 13C NMR chemical shifts of 48 industrially preferred solvents in six commonly used deuterated NMR solvents (CDCl3, acetone-d6, DMSO-d6, acetonitrile-d3, methanol-d4, and D2O) are reported.Thus, the left part of the chart is the low-field, or downfield, side, and the right part is the high-field, or upfield, side. The peak at just less than 170 is the carbon in a carbon-oxygen double bond. Gasteiger, “ Prediction of 1H NMR Chemical Shifts Using Neural Networks ”, Analytical Chemistry , 2002, 74 (1), 80-90. Over the past fifty years nuclear magnetic resonance spectroscopy, commonly referred to as nmr, has become the preeminent technique for determining the structure of organic compounds. You can also simulate 13C, 1H as well as 2D spectra like COSY, .Spin-Spin splitting .NMR acquisition, this pulse sequence will be repeated many times in order to improve the signal-to-noise ratio (S/N), which increases with the square root of the number of transients that are averaged together (nt).
NMR Spectroscopy- Definition, Principle, Steps, Parts, Uses
Online chemical shift tables and charts. The 13 C-13 Cspin-spin splitting rarely exit between adjacent carbons because 13 C is naturally lower abundant (1.3 R 3 CH 3˚ aliphatic 1.


1H number of signals. Here we present the NMR shifts of the most commonly used solvents and impurities in organic synthesis measured in the 7 most frequently used .Journal of Magnetic Resonance 2011.Nuclei that absorb on the downfield side of the chart require a lower field strength for resonance, implying . 1H NMR spectra of Boc amino acids.1ppm whereas CH2Cl2 = 5. Of all the spectroscopic methods, it is the only one for which a complete analysis and interpretation of the entire spectrum is normally expected. Simulate and predict NMR spectra directly from your webbrowser using standard HTML5. This is because the signals for some types of carbons are inherently weaker than for other types – peaks corresponding to carbonyl carbons, for example, are much smaller than .0 70 40 95 80 60 30 70 40 80.NMR spectroscopy is the use of the NMR phenomenon to study the physical, chemical, and biological properties of matter.64 ︎ T2 : 52.0 license and was authored, remixed, and/or curated by LibreTexts. Aires-de-Sousa, M.
NMR Spectroscopy
These tables can support you in identifying and separating NMR signals of impurities that might originate from residual solvents or from your reaction apparatus. The user can independently set each of the parameters shown in Figure 1.3 • Chemical Shifts NMR spectra are displayed on charts that show the applied field strength increasing from left to right (Figure 13.
1H NMR Tables
Comparing the 1 H NMR, there is a big difference thing in the 13 C NMR. For example, NMR can .2–3 C=C–C H .NMR charts are calibrated using an arbitrary scale called the delta (δ) scale, where 1 δ equals 1 part-per-million (1 ppm) of the spectrometer operating frequency.0 220 200 180 160 140 120 100 80 60 40 20 0 ppm Alcohols Ethers Substituted Benzenes Alkenes Carbonyl: Ester Amide Carboxylic Acid Carbonyl: Aldehyde Ketone Alkanes Alkynes Amines Alkyl bromides Alkyl chlorides Alkyl . This re-orientation occurs with absorption of electromagnetic radiation in the radio . Overview of typical 1H NMR shifts.Part 1: Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy) – An Overview. Note: alkene region modified from earlier handout. Richard Spinney (The Ohio State University) 12. It is an analytical chemistry technique used in quality control.3 R 3 C H 3˚ aliphatic 1. 2) RSI resistance breakout. 1H NMR integrate and find the structure. Knowledge of their purpose and function will help you obtain quality .30% in the last 24 hours. 13 C-1 H Spin coupling: 13 C-1 H Spin coupling provides useful information about the number of protons attached a carbon atom.Table of characteristic proton NMR chemical shifts. type of proton type of compound chemical shift range, ppm RC H 3 1˚ aliphatic 0.Basic NMR Concepts: A Guide for the Modern Laboratory Description: This handout is designed to furnish you with a basic understanding of Nuclear Magnetic Resonance (NMR) Spectroscopy. Unknown molecule: First, if the molecular formula is known, then start by calculating the degree of . 1H NMR spectra of small molecules.The Basics of 13 C-NMR spectroscopy. A number of reasons now exist to be long, including: 1) The ‘incredible buy’ signal has printed. You may find more information on the authors website. and does not require JAVA (only HTML5)!!! This page allows to predict the spectrum from the chemical structure based on Spinus. 1 shows 13 C NMR spectrum for three related molecules: p -nitrophenol, o -nitrophenol, and m -nitrophenol. The following steps summarize the process: Count the number of signals to determine how many distinct proton environments are in the molecule (neglecting, for the time being, the possibility of overlapping signals). Chemical Formula: C 5 H 9 ClO.1H exercise generator. 4) On the 3-day line char.Sehen Sie Krypto-Kurse und Charts live von Numeraire, Marktkapitalisierung, 24-Stunden-Handelsvolumen von NMR, zirkulierendes Angebot, neueste Nachrichten und mehr.NMR resurrect Try the new HTML5 only predictor that works also on iPad, Android, .
The chemical shift is the resonant frequency of a nucleus relative to a standard in a magnetic field (often TMS). 3) Points 1 and 2 are also true for the BTC pair (below).We update our NMR to USD price in real-time.

Predict 1H proton NMR spectra
The most extensive collection of chemical shifts for 1H, 13C, 19F, 31P and 77Se (Hans Reich, Univ of Wisconsin) CIL solvent NMR data chart and storage information; 1H chemical shifts; 13C chemical shifts; 19F chemical shifts (Compiled by Indiana University NMR Facility) Molecular weight, density . (2) Schematic diagrams of NMR chemical shift data for 13C.They show the typical chemical shifts for protons being influenced by a single group. It has a circulating supply of 6,273,703 NMR coins and .Common Solvent Peak Coupling and Chemical Shift Values.
Simulate and predict NMR spectra
14: Predict the structures of A and B in the following reaction: Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris) 9. Note again the additive effects of multiple attached groups.69 ?Stop Trigger: • If you make capital management: Stop is activated when one candle opening and closing below the stop level of the same time frame of the signal. Enter TROSY Relaxation Rate (Hz): . It also includes NMR summary data on coupling constants and chemical shift of 1H, 13C, 19F, .9 R 2 C H 2 2˚ aliphatic 1. • If you don’t make capital manage.3: Chemical Shifts and Shielding is shared under a CC BY-NC-SA 4. The peak at just over 50 must be a carbon attached to an oxygen by a single bond.NMR/USDT I BUY SETUP ︎ ? BINANCE:NMRUSDT SIGNAL ︎ENTRY : market ? TARGETS : ︎ T1 : 46. Numeraire is down 5. For Conversion of TRACT data to τ c.
- Nitro 24 Stunden Rennen Heute , 24h Nürburgring Ticker-Nachlese: Stimmen zum Frikadelli-Sieg
- Norddeutsche Staaten Beziehungen
- Nivea Powder Touch _ Nivea Powder Touch Antyperspirant
- Nikon Lenses Latest Version _ Update Your Nikon Z Lens & FTZ Firmware
- Ninjago Videospiele Kostenlos | The LEGO NINJAGO Movie Video Game
- Noni Rücksendung | Rücksendezentrum
- Nino Tempo , Nino Tempo & April Stevens music, videos, stats, and photos
- Nissan Juke Hybrid 2024 Bilder
- No Angels Tour 2024 Deutschland
- Nikotin Beruhigende Wirkung – Diese 7 Kräuter wirken beruhigend
- Normalverteilung Online Rechner
- Nombres De Caballos Famosos _ Nombres para caballos y yeguas 【2022】
- Normales Radio An Schüssel Anschließen
- Nmap Port Scan Command Example
- Nintendo 3 Ds Xl Preis : Nintendo Ds, Konsole gebraucht kaufen