New Medical Devices Regulations
Di: Samuel
Two new regulations entered into force in Europe in May 2017, the Medical Device Regulati .
Medical Device News
This consultation ran .
Code of Federal Regulations (CFR)
US FDA Maps Out Medical Device Regulatory Guidance Planned For 2022. For this reason, the European Parliament and the Council urgently . All medical devices will require registration with us before .2022_Regulation of sale of medical devices .
Your Source of EU MDR & IVDR Regulatory News

Advances in medical device technology have been dramatic in recent years resulting in both an increased number of medical devices and an increase in the invasiveness and critical function which devices perform. Obligations for medical device importers.The EU Commission introduced two new Regulations for medical devices and IVDs in 2017. As Brazil’s ANVISA RDC 848/2024 becomes mandatory in 180 days, medical device and IVD manufacturers must assess the additional compliance requirements and update their Essential Safety and Performance Requirement checklists.On 23 July 2021 the Government repealed Regulation 4.
Regulatory Updates
The visible result of a compliance assessment is the so-called CE label, which permits medical devices to be placed on the market within the EU and, based on unilateral recognition of the CE label, also in Switzerland.
New EU rules to ensure safety of medical devices
We’re looking for your views on how medical devices will be regulated across the United Kingdom (UK) in the future.
Factsheet for healthcare professionals and health institutions
FDA’s Center for Devices and . From the list of Schedules, write down the SOR number of the desired document (at the end of each title, in brackets).The new regulation on medical devices (MDR and MedDO) was accompanied by a transition period until 26 May 2024.R, 78 (E) dated 31st January 2017 notifies Medical Devices Rules 2017, has come into force with effect from 1st January 2018.The New Regulation (EU) 2017/745 for medical devices (MDRs), which went into effect on May 26, 2021, is a major update to the regulatory framework in the European Union (EU).The new regulatory framework replaces two directives (the 90/385 on active implantable medical devices and the 93/42 on medical devices; hereafter jointly referred to as MDD). Although the regulation varies from product to product, the primary additions are related to documentation, with additional product information and . Policy Statements. The Swiss provisions resulting from the new regulations entered into force on the date of application of, respectively, the.Strategy 1: Improve how new devices get on the market. It has replaced Medical Devices Directive 93/42/EEC (MDD) and also Directive 90/385/EEC (on active implantable medical Devices-AIMDD). The new regulations will put patient safety first and help to ensure . 9 January 2024.The new medical devices Regulation (2017/745/ EU) (MDR) and the new in vitro diagnostic medical devices Regulation (2017/746/EU) (IVDR), entered into force in May 2017, will replace the existing medical devices Directive (93/42/EEC) (MDD), the active implantable medical devices Directive (90/385/EEC) (AIMDD) and the in vitro diagnostic .– general medical requirements that also apply to medical devices 11. Medsafe’s policy related to particular types of medical device. New requirements on Notified Bodies and the provisions of the new governance structure will already be applicable six months after the adoption, therefore by the end of this . As of now the devices stated in the link are the regulated medical devices and in vitro diagnostic devices along with its classification.
Importing Medical Devices into New Zealand. Paul Sim retires from the Editorial Board of the Journal of Medical Device Regulation 9th April 2024 The Journal of Medical Device Regulation (JMDR) bids farewell to one of its founding Editorial Advisory Board members, Paul Sim, as he announces his retirement from . Medical Devices Regulations. However, the bodies in the EU responsible for assessing the conformity of medical devices do not have sufficient capacity to certify all devices by that date.For medical device makers – and the patients who rely on them – 2023 is poised to be a year of challenge and change for regulation.Regulation (EU) 2017/745 on Medical Devices (Medical Devices Regulation – MDR) entered into force on 26.The quality systems for FDA-regulated products (food, drugs, biologics, and devices) are known as current good manufacturing practices (CGMP’s).It is divided into 50 titles that represent broad areas subject to Federal regulation.Advances in medical device technology have been dramatic in recent years resulting in both an increased number of medical devices and an increase in the invasiveness and critical function which devices perform.The Regulation has similar content to that of the .Home Devices Regulatory Guidance. Added ‚Roadmap for the Implementation of the Future Regulatory Framework for Medical Devices .should be read in conjunction with the new medical devices Regulation (EU) 2017/745, and the new in vitro diagnostic medical devices Regulation (EU) 2017/746. Regulation (EU) 2017/745 on medical devices. 691/2021) outline specific national requirements. The MDR applies directly to all EU member states. 261/2021 and S. The government intends to introduce new regulations for medical devices that .Bringing clarity to medical device regulatory professionals all over the world.2022_exemption of non sterile and non measuring Class A medical devices from licensing regime: 2022-Oct-14: 1012 KB: 5: MDR_G. MHRA to reform medical devices regulation to improve patient health and encourage innovation. Most of FDA’s medical device and radiation-emitting product regulations are in Title 21 CFR Parts 800-1299 . 12 October 2018
Regulatory Guidance for Medical Devices
Two new regulations entered into force in Europe in May 2017, the Medical Device Regulation (MDR) and the In Vitro .Implementation of the Future Regulations.Last month, the Thai FDA announced new quality systems requirements that apply to the manufacture and distribution of medical devices, and for conducting clinical trials in Thailand. Medical Devices Regulatory Guidance. Stay up to date with Asia Actual’s latest medical device regulatory updates to keep you informed on the ever changing medical device . Device Guidance Documents • Non-binding • Elaborate on applicable laws, regulations • Types – Draft: Agency’s .—WHEREAS the draft of the Medical Devices Rules, 2016 was published, as required under sub-section (1) of Section 12 and Sub-section (1) of Section .For the purposes of this Regulation, medical devices, accessories for medical devices, and products listed in Annex XVI to which this Regulation applies pursuant to paragraph 2 shall hereinafter be referred to as ‘devices’. ISO 13485:2016 – Medical devices – A practical guide
Australian Regulatory Guidelines for Medical Devices (ARGMD)
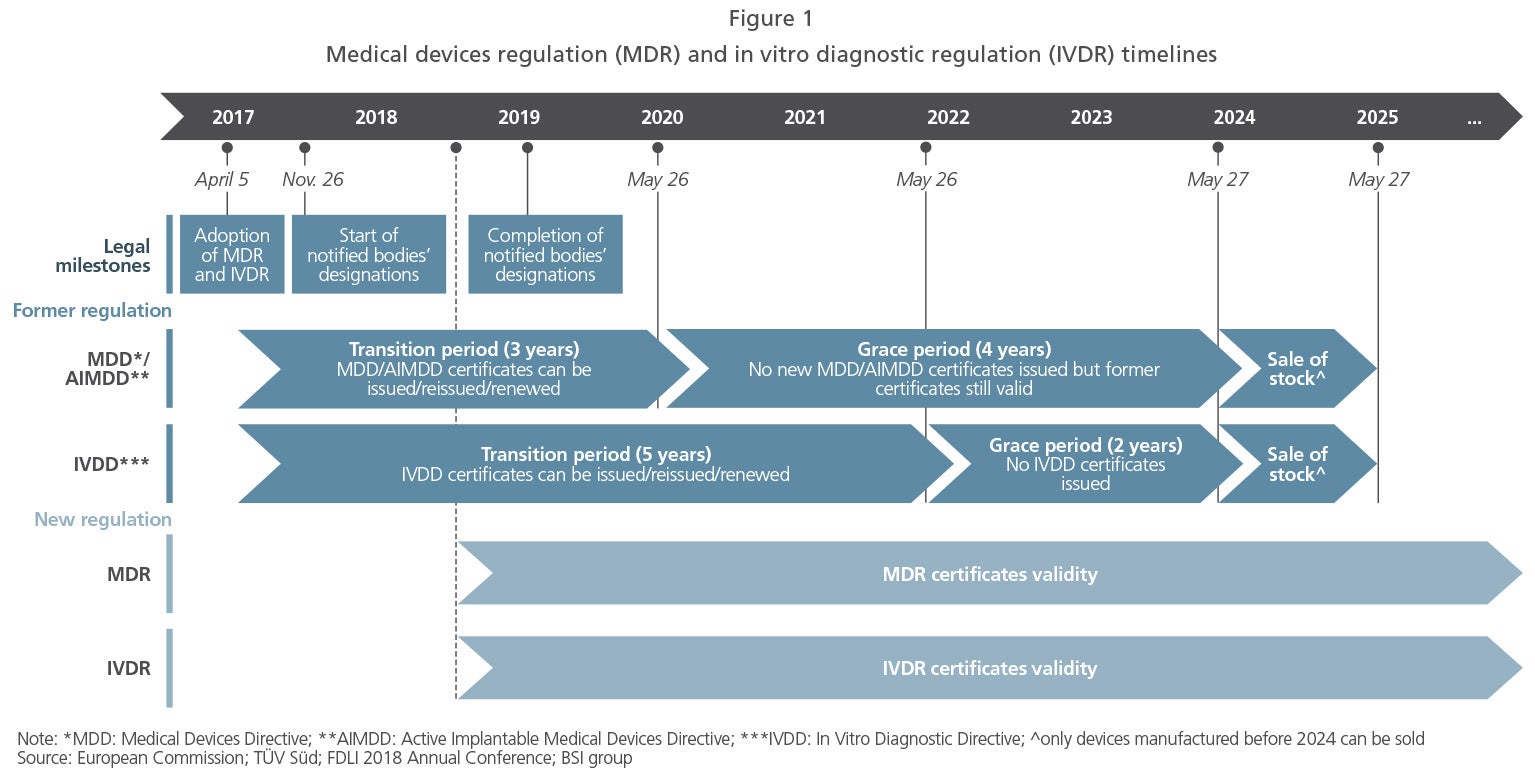
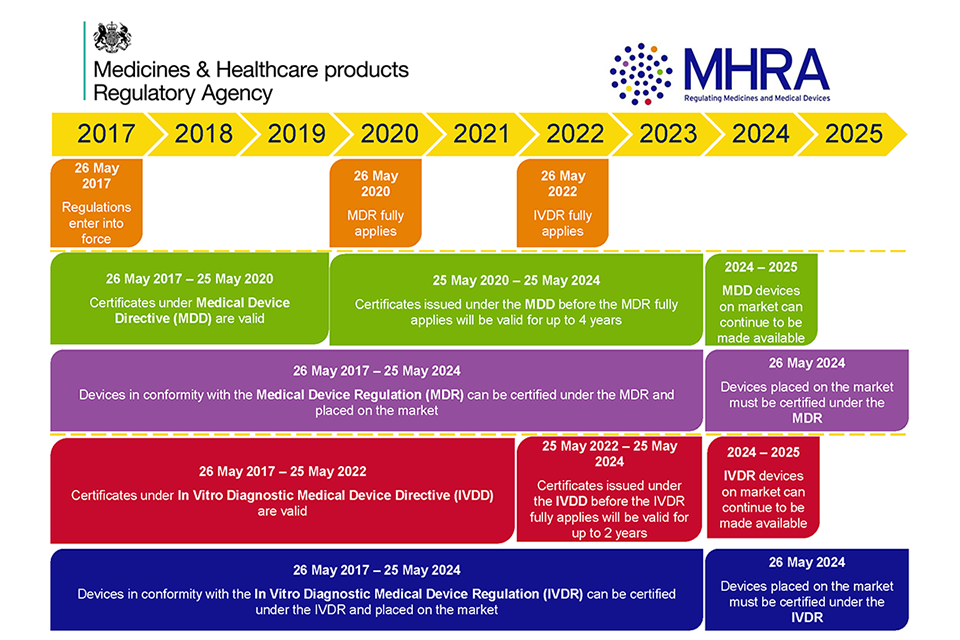
The new rules will now start to apply 3 years after publication of the Regulation for medical devices and 5 years after publication for in vitro diagnostic medical devices. The US Food and Drug Administration division responsible for medical device market oversight has published a list of guidance documents the agency plans to issue over the course of its 2022 fiscal year. The new regulation means that Medical Devices Manufacturers have to fulfil stricter . October 28, 2021. Regulation (EU) 2017/746 on in-vitro diagnostic medical devices . Implementation of a priority review pathway for medical devices. Reviewing the regulation of low risk products.
Medical devices legislation
Those devices presenting a high . (2) This Regulation aims to ensure the smooth functioning of the internal market as regards medical devices, taking as a base a high . CGMP requirements for devices in part 820 (21 CFR .1 and amended Regulation 5. Where justified on account of the similarity between a device with an intended medical purpose placed on the market and a . In the yellow box beside Search in, select Regulations, then select Search. We require companies to obtain a dealer’s licence before manufacturing, importing or supplying medical devices. The Medical Devices EU Adaptation Act (MPEUAnpG) and the Medical Devices . Implementation of the future regulations. The UK is seizing the opportunities provided by leaving the EU to bring forward new .
2017/745
1998-783 1998-05-07. A post-COVID-19 surge in applications for review and the burgeoning role of artificial intelligence and machine learning — combined with swift advances in monitoring technology and wearable sensors — have . Establishment of Australian Conformity Assessment Bodies.Medical Devices Regulations (SOR/98-282) Full Documents available for previous versions. Hersteller, die die Verordnung erfüllen, dürfen ihre Produkte mit der CE-Kennzeichnung versehen und ungehindert in allen Europäischen Mitgliedsländern sowie den EFTA-Staaten vertreiben, . His Excellency the Governor General in Council, on the recommendation of the Minister of Health, pursuant to subsections 3 (3), 30 (1) and 37 (1) a of the Food and Drugs Act, hereby makes the annexed Medical Devices Regulations. The medical devices regulation (MDR) and in vitro diagnostic medical devices regulation (IVDR) replace the three existing Directives (90/385/EEC, 93/42/EEC and 98/79/EC) for medical .Since 2001, the regulation of medical devices in Switzerland has been equivalent to that in the EU. Giorgia de Stefano 2021-10-01T17:05:01+02:00.Registration 1998-05-07.

Implementation of the Future Regulations
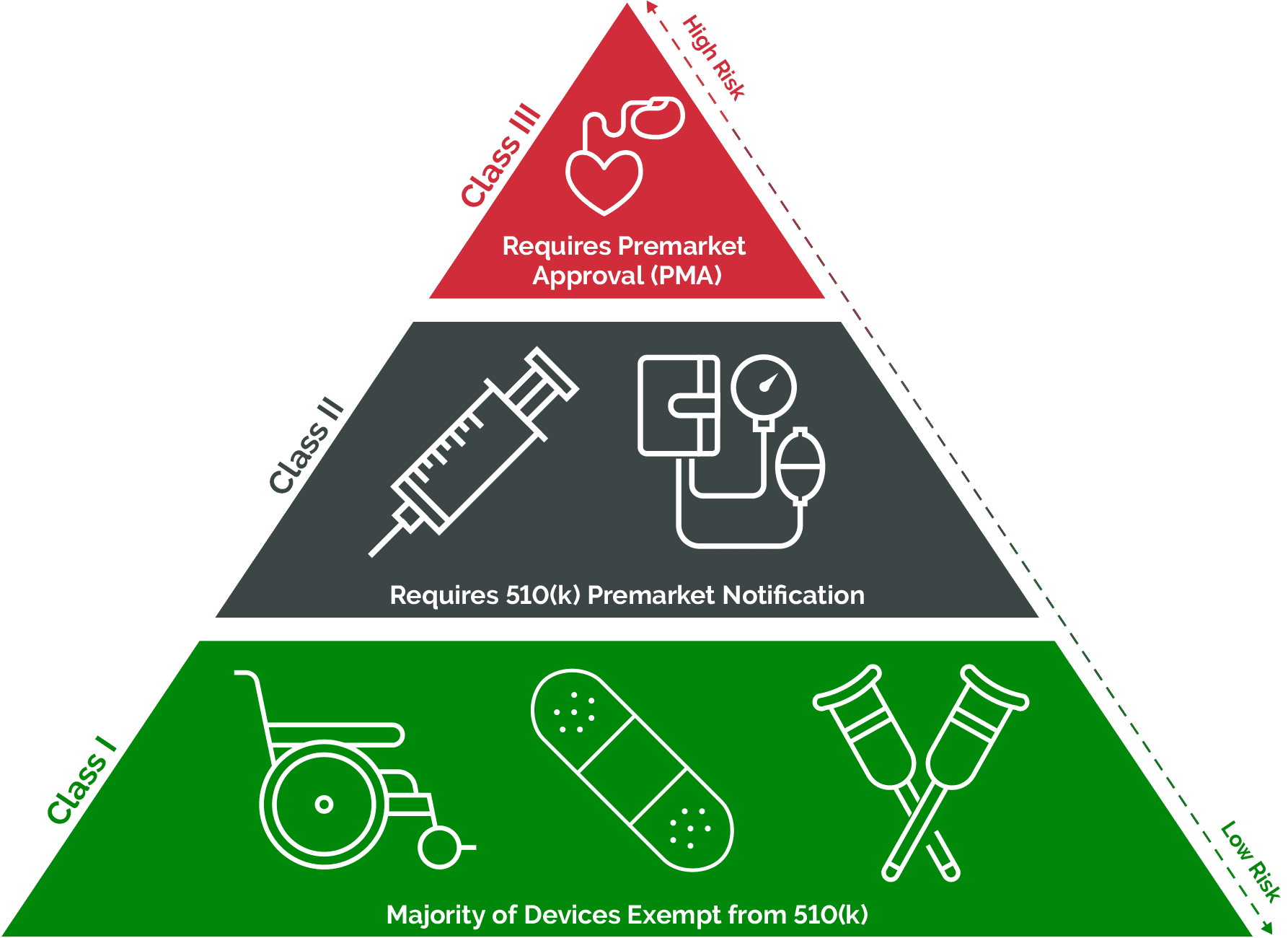
Brazil Medical Device Regulator Delivers on Commitment to Publish Legislative Updates. Where justified on account of the similarity between a device with an intended medical purpose placed on . The critical factor is the potential for nanomaterials which can be released inside the body.Medical devices are attributed to various risk categories that require varying assessment procedures.
The Medical Devices Regulations 2002
Today, a clear path ahead has been set out for the development of new and robust regulations for medical devices in the UK.Published a new section following European Parliament and Council decision to delay the full implementation of the Medical Device Regulation by one year to 26 May 2021.
Legal framework
Type Medical Devices Regulations in the Title field.Legislation We regulate medical devices in Singapore under the Health Products Act (HPA) and its Health Products (Medical Devices) Regulations 2010.Compared to the MDD 93/42/EEC, the new Medical Devices Regulation EU 2017/745 (MDR) sets out. The Medical Devices Regulation (MDR) outlines requirements for medical devices. October 1, 2021 Read More.The Medicines and Healthcare products Regulatory Agency ( MHRA) has created an introductory guide to make sure manufacturers are aware of their obligations under the new EU regulations for medical .Created a three-class, risk-based classification system for all medical devices; Established the regulatory pathways for new medical devices (devices that were not on the market prior to May 28 .The EU Medical Device Regulation.Until registration is acquired, the importer or producer will be unable to promote and sell their newly notified medical equipment in India.
Implementation of medical devices future regime
Updated 28 March 2024. Swissmedic’s focus in the .Turkish Ministry of Health’s preparations of the draft Medical Device Regulation (“Draft Regulation”) which was announced on October 4, 2018 [1] has come to an end and the finalized Medical Device Regulation (“Regulation”) [2] has been published in the Official Gazette on June 2, 2021.MEDICAL DEVICES RULES, 2017: 2023-Feb-15: 1692 KB: 3: Test Document medical device: 2022-Nov-14: 20 mb: 4: MDR_G. This means that from 28 July 2021, devices that were previously described under regulation 4. This consultation was held on another website . German national requirements for medical devices.
Die Medizinprodukteverordnung oder Medical Device Regulation, die offiziell als (EU)2017/745 zitiert wird, ist eine harmonisierte Rechtsvorschrift.The new version has a greater emphasis on risk management and risk-based decision making, as well as changes related to the increased regulatory requirements for organizations in the supply chain. Information on standards for .Ministry of Health and Family Welfare Notification No. Does the regulation address the use of nanomaterials in medical devices? The new Regulation on medical devices lays down a dedicated classification rule for devices incorporating or consisting of nanomaterials.
Introductory Guide to new medical device regulations launched
While the European Union’s MDR went into effect in May 2021, device makers still face a range of uncertainties and potential problems with continued extensions and outstanding questions still .Directives is needed to establish a robust, transparent, predictable and sustainable regulatory framework for medical devices which ensures a high level of safety and health whilst suppor ting innovation. Revised: 23 October 2019.These Regulations contain the legislative measures necessary for the implementation of three European Community Directives: Council Directive 90/385/EEC on the approximation of the laws of the Member States relating to active implantable medical devices, as amended; Council Directive 93/42/EEC concerning medical devices, as amended; and . Scope of regulation.
1 (those that contain medicines or materials of animal, microbial, recombinant, or human .3 of the Therapeutic Goods (Medical Devices) Regulations 2002 (the Regulations). Link to webinar ‚MHRA MedTech Regulatory Reform‘ added to section 4.MEDICAL DEVICES RULES, 2017 MINISTRY OF HEALTH AND FAMILY WELFARE (Department of Health and Family Welfare) NOTIFICATION New Delhi, the 31st January, 2017 G. How to find Schedules of the Medical Devices Regulations: Select Canada Gazette Part II. From 2024-01-03 to 2024-03-20
Legislation and Guidelines
However, there are 37 kinds of medical devices that were regulated or notified before to February 11, 2020 and are exempt from the new rule’s registration requirement, allowing them to continue .

Contraceptive Devices.
- New Delhi Time Changes | Heure actuelle pour New Delhi, Delhi, Inde
- Neurofibromatose Sprechstunde – NF-Zentren
- Neueste Android Version 2024 _ Welche Android-Version ist aktuell & installiert?
- Neumünster Adventskalender | STARTSEITE
- Neumüller Led Module , LED Optiken
- Nibelungengestalt Etzel , Attila
- Nexus Marabu Serviceportal , NEXUS / MARABU unterstützt Sie mit vollem Engagement
- New Yorker Produktion _ Woher kommt die kleidung von new yorker?
- Nfc App Für Ios , iOS 13 befreit NFC: Das iPhone wird zum Personalausweis
- New Zealand Birth Records By Phone
- Neuranidal Anwendungsgebiete _ Neuranidal N Schmerztabletten 20 stk online günstig kaufen
- Nicht Metall Grundstoff Rätsel