Nacl Structure : Chlorure de sodium
Di: Samuel
CsCl CsCl and NaCl NaCl do not adopt identical crystal packing arrangements because the Cs+ Cs + is considerably larger than the Na+ Na + ion.
The edge length of the cubic unit cell of NaH is 4. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.
La structure NaCl
In this video we’ll look at the crystalline structure of NaCl (Sodium chloride). Cl¹⁻ is bonded in a body-centered cubic . Calculate the ionic radius of H −. Le chlorure de sodium a une structure cristalline constituée d’un arrangement régulier d’ions sodium et chlorure dans un rapport 1:1. Other names: Salt Permanent link for this species. Quindi è una molecola biatomica.
Chemistry of Salt (NaCl)
These compounds include: FeS 2 (pyrite, fools gold): S 22- (disulfide) and Fe 2+. Dans la structure de NaCl Lewis, le métal Na donne son 1 électron à l’atome de Cl pour former une liaison ionique.
mp-22862: NaCl (cubic, Fm-3m, 225)
CAS Registry Number: 7647-14-5. All Na–Cl bond lengths are 3. On l’appelle plus communément sel de table ou sel de cuisine, ou tout simplement sel dans le langage courant.
Natriumchlorid-Struktur
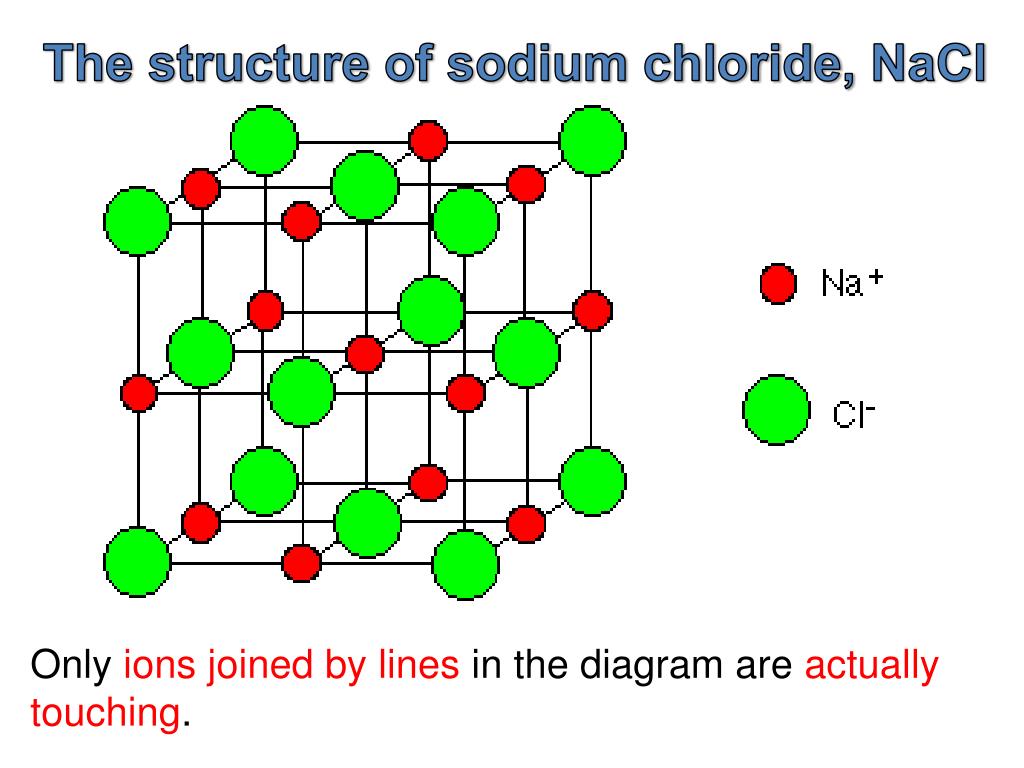
NiAs structure.Dichte Packungen; NaCl-Typ.Sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Na1+ is bonded to six equivalent Cl1- atoms to form a mixture of edge and corner-sharing NaCl6 octahedra.In solid sodium chloride, each ion is surrounded by six ions of the opposite charge as expected on electrostatic grounds. The rocksalt or NaCl structure is a very common structure among binary ionic compounds. den Natriumchloridtyp. This structure can be visualized in three dimensions as “balls” with interconnecting “sticks. It is well established that NaCl has a B1 (“rock-salt”) structure that transforms .Sodium chloride, NaCl, is an ionic crystal. die meisten Erdalkalioxide wie MgO. About 1% to 5 % of seawater is made of NaCl. The Materials Structure Interactive Gallery is a collaboration of the research from faculty, graduate students, and . È un sale composto da due soli atomi. Alle Oktaederlücken sind komplett mit Na-Ionen gefüllt (N Atome = N Oktaederlücken = Zusammensetzung AB). Le chlorure de sodium (sel) composé chimique ionique de formule NaCl structure cristalline d’ions Na+ et Cl– de maille cubique.Structure cristalline du chlorure de sodium. This compound is water-soluble and consists of sodium cation and .The edge length of the unit cell of LiCl (NaCl-like structure, FCC) is 0. Die Natriumchlorid-Struktur ( Kochsalzstruktur) bzw. 3: In a cesium chloride crystal, the cesium ion (orange) occupies the center, while the chloride ions (green) occupy each corner of the cube. Im NaCl-Typ bilden die Cl-Ionen eine kubisch dichteste Kugelpackung. Although it may be a little .The structure of CsCl can be seen as two inter penetrating cubes, one of Cs + and one of Cl-.Die Struktur des grauen Arsens bzw. For example, crystals of cubic rock salt (NaCl) are physically cubic in appearance. posted: January, 2015. La géométrie du chlorure de sodium est octaédrique, c’est-à-dire que chaque atome de sodium est entouré de 6 ions chlorure et vice versa. des Germaniumtelluridtyps stellen eine verzerrte Kochsalzstruktur dar.
Crystal Structure of NaCl : Bond, Ionic and Covalent
C’est le plus gros composant du sel comestible et sa forme minérale est connue sous le nom d’halite. The molecular weight of NaCl is 58.Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Dans sa structure de Lewis, Na est lié à Cl avec une liaison ionique. Since there are 4 atoms or ions present in each unit cell of a face centred cubic structure, therefore, the number of NaCl units in a unit cell of NaCl is four. Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript . Beispiele für Vertreter dieses Strukturtyps sind neben Natriumchlorid selbst Lithiumchlorid (LiCl) und Kaliumchlorid (KCl), aber auch die meisten Erdalkalioxide, wie MgO. Sodium ions alternate with chloride ions in a three- dimensional pattern. The structure is three-dimensional. Sie ist für viele Salze typisch, weitere Vertreter dieses Strukturtyps sind z. V přírodě se vyskytuje v podobě nerostu halitu, známého též pod názvem sůl kamenná. Thallium(I) iodide crystallizes with the same structure as CsCl. La valeur de sa constante de réseau est 564. Le chlorure de sodium a un centré sur le visage réseau cubique comme sa structure cristalline. (The ionic radius of Li + is 0. Ainsi, en raison de la perte d’électrons, des charges positives et négatives se créent sur les atomes de Na et de Cl. Natriumchlorid ist für Menschen und Tiere der wichtigste Mineralstoff.Seine hauptsächliche Anwendung ist neben dem direkten Einsatz als Oxidationsmittel die Herstellung von Chlordioxid, da dieses zu instabil für Transport und Lagerung ist.Natriumchlorit mit der Summenformel NaClO 2 – nicht zu verwechseln mit dem Kochsalz Natriumchlorid (NaCl) – ist das Natriumsalz der Chlorigen Säure.In this video, we’ll explore the crystal structure of Sodium Chloride, also known as table salt, and learn how to draw its crystal lattice using an easy and . Cl1- is bonded to six equivalent Na1+ . Die Struktur des schwarzen Phosphors bzw.Table of contents. La structure du sel peut être décrite standard le contenu de sa maille.Sodium chloride is also known as salt. Every tiny grain of sodium chloride contains billions of sodium ions and chloride ions. Use this link for bookmarking this species for future reference. Assuming that the lithium ion is small enough so that the chloride ions are in contact, calculate the ionic radius for the chloride ion. All Na–Cl bond lengths are 2. Information on this page: Gas phase thermochemistry data; Condensed . The chlorine lacks one electron to fill a . Die dichtesten Ebenen verlaufen senkrecht zu allen Raumdiagonalen der kubischen Elementarzelle.The XRD analysis shows that the salt has face centered cubic (FCC) structure with average crystallite size of 70.Structure refers to the internal arrangement of particles and not the external appearance of the crystal.NaCl is Tetraauricupride structured and crystallizes in the cubic Pm-3m space group.
Strukturtypen-Datenbank
The first atom is located at each lattice point, and the second atom is located halfway between lattice points along the fcc unit .
sodium chloride
14 electron volts of energy to remove that electron. The 6 closest neighbors to the Cl-ion marked with an arrow are 6 Na + ions. The corner-sharing octahedral tilt angles are 0°. It can be represented as a face-centered cubic (fcc) lattice with a two-atom basis or as two.NaH crystallizes with the same crystal structure as NaCl.34 g Na and 60. Note: The length unit angstrom, Å, is often used to represent atomic-scale dimensions and is equivalent to 10 −10 m. Jedná se o velmi důležitou sloučeninu potřebnou pro životní funkce většiny organismů . Na1+ is bonded in a body-centered cubic geometry to eight equivalent Cl1- atoms.
Chlorure de sodium
The pattern in the sodium chloride is simple. Thalliumiodidtyp.45 g/mol respectively, 100 g of NaCl contain 39.407 Å Z=4; Motif périodique : FeS 2 (type AX) S 2 2-est un ion double: Structure de la pyrite de fer : FeS 2: Positions atomiques Fe 2+ 0 , 0 , 0 S 2 2- ½ , 0 , 0 : Retour Dernière mise à jour : .
X-ray diffraction (XRD) pattern of NaCl salt
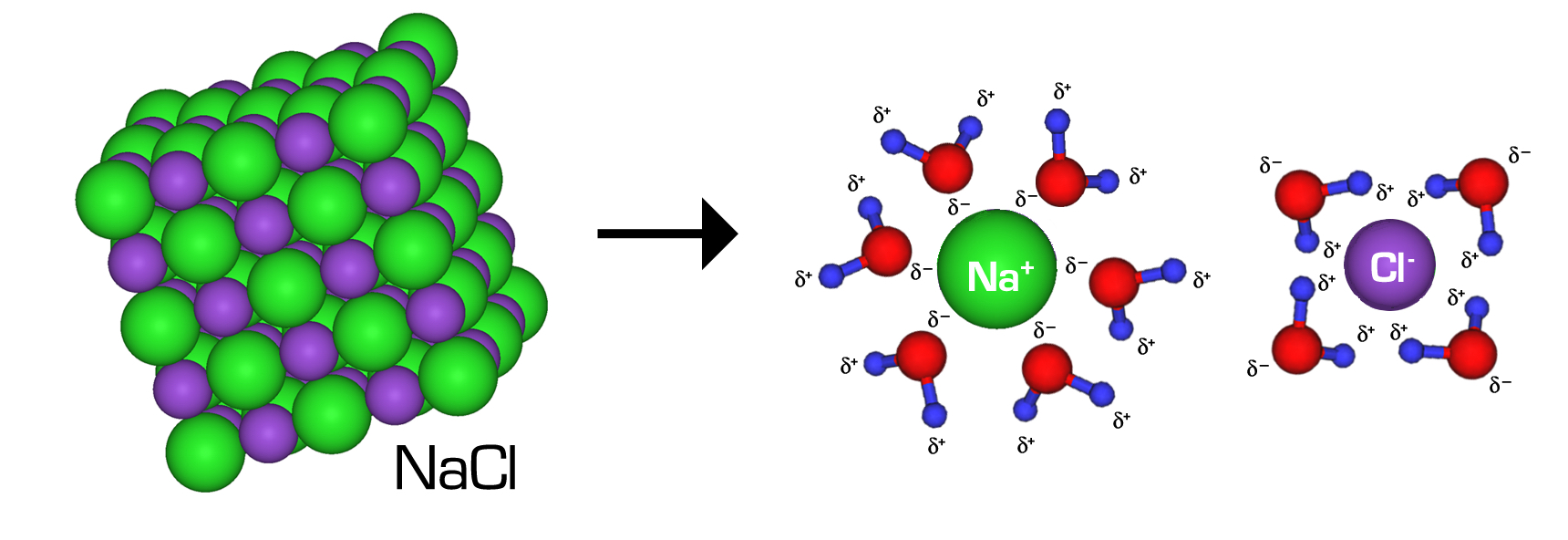
Es ist das Mittel der . It occurs in oceans and sea waters. The closest neighbors to the Cl-ion marked with an arrow are 6 Na + ions. Touching would cause repulsion between the anion and cation.Sodium Chloride (NaCl) The sodium chloride structure adopts a face-centered cubic lattice with a two-atom basis or as two interpenetrating face centered cubic lattices. It is a crystalline solid, white. Na¹⁺ is bonded in a body-centered cubic geometry to eight equivalent Cl¹⁻ atoms. La struttura di lewis di NaCl coinvolgeva principalmente 2 elementi, ovvero l’atomo di metallo di sodio (Na) e cloro (Cl). Ihr Prototyp ist die Kristallstruktur von Natriumchlorid (Kochsalz, Halit). However, these are not entirely independent since the external appearance of a crystal is often related to the internal arrangement. The ions are not touching one another.Lorsque le NaCl se dissout, sa structure cristalline tombe en panne, et les ions individuels s’hydrater. È una combinazione di metallo (Na) e non metallo (Cl).
Structure et caractéristiques du NaCl Lewis : 19 faits complets
Which structure a simple 1:1 compound like NaCl or CsCl crystallizes in depends on the radius ratio of the positive and the negative ions. It is also found as rock salt.
Sodium Chloride, NaCl
La densité d’une solution de NaCl augmente à mesure que du NaCl se dissout dans l’eau, en raison de la masse ajoutée sans un changement important en volume.This NBC News Learn video explains and illustrates the molecular structure of sodium chloride (NaCl) crystals; the structure and symmetry of crystal lattices. NaCl has four cations and four anions in a face-centered cubic unit cell.” The balls are Na + and Cl-ions.Representation of the structure of NaCl.Crystal structure and Unit Cell. CaC 2 (a salt-like carbide): Ca 2+ and linear C 22- anions.NaCl is Tetraauricupride structured and crystallizes in the cubic Pm̅3m space group.更新 2024-2-23 塩化ナトリウム型構造(岩塩型構造、NaCl型構造、NaCl-type structure、rock salt structure) 塩化ナトリウム型構造は、の組成で表される二元系物質の多くに見られる結晶構造であり、三次元空間に原子をチェッカーボード状に並べたような配置をしています. Because NaCl is an ionic compound we don’t talk about molecular geometry or.This structure consists essentially of a FCC (CCP) lattice of sulfur atoms (orange) (equivalent to the lattice of chloride ions in NaCl) in which zinc ions (green) occupy half of the tetrahedral sites. The first atom is located at each lattice point, and the second atom is located half way between lattice points along the face-centered cubic unit cell edge. The salient features of its structure are:Chlorid sodný ( NaCl) je chemická sloučenina běžně známá pod označeními kuchyňská sůl, jedlá sůl nebo ještě častěji jen jako sůl. Sa formule moléculaire est NaCl, et il décrit la proportion stoechiométrique de ses ions (Na . Auch beim grauen Selen und beim metallischen Tellur besteht eine verzerrt oktaedrische Anordnung der Atome. The edge length of the unit cell of TlI is 4. All Na-Cl bond lengths are 3. In its aqueous form, it is called a saline solution.Sodium Chloride, NaCl The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. der Natriumchloridtyp ist einer der Strukturtypen der Kristallographie.C’est donc une molécule diatomique. CsCl is more stable than NaCl, for it produces a more stable crystal .Les différentes représentations de la structure NaCl; Exemple d’une structure dérivant de la structure NaCl: FeS 2 Pyrite Motif formulaire : FeS 2; Maille : a = 5.Penn State University via Wikibook.1 Rocksalt (NaCl) Structure. The coordination number for both ions is 8. Some may mistake the structure type of CsCl with NaCl, but really the two are different. NaCl is a structure consisting of two interpenetrating FCC lattices, where larger chlorine atoms occupying the face-centered cubic position with the smaller sodium ions are placed in the octahedral void (outline . Other names: Salt. As with any FCC lattice, there are four atoms of sulfur per unit cell, and the the four zinc atoms are totally contained in the unit cell. There are a number of compounds that have structures similar to that of NaCl, but have a lower symmetry (usually imposed by the geometry of the anion) than NaCl itself.
mp-22851: NaCl (cubic, Pm-3m, 221)
Crystals of sodium chloride have a regular, cubic structure.Le chlorure de sodium, également appelé sel commun ou sel de table, est un sel inorganique binaire du sodium de métal alcalin et du chlore halogène. Permanent link for this species. When this structure was originally solved (in 1913 by using X-ray diffraction) by W.Structure du chlorure de sodium.
John – Wikimedia . Ionic bonds hold the ions tightly. updated: February, 2015.With molar masses of 22. Cette roche évaporite a l’aspect d’une matière cristalline, sèche et solide, de densité 2,2, de dureté Mohs 2 et surtout friable, très soluble dans l’eau, avec un goût . If the radius of the positive ion is bigger than 73% of that of the negative ion, then 8:8-coordination is possible.Der Körper eines erwachsenen Menschen enthält etwa 150–300 g . La structure est un réseau cubique à faces centrées, avec chaque ion sodium entouré de six ions chlorure et chaque ion chlorure entouré de six ions sodium.Questo editoriale mostra uno sguardo approfondito su di esso di seguito.

Die für viele Salze typische kubisch-flächenzentrierte Kristallstruktur von Natriumchlorid (Kochsalz) ist Prototyp für die Natriumchlorid-Struktur bzw. Bragg, his interpretation met resistance by chemists who thought that precise integer stoichiometries were a consequence of the . NaCl has a cubic crystal system and a face-centered cubic crystalline structure.
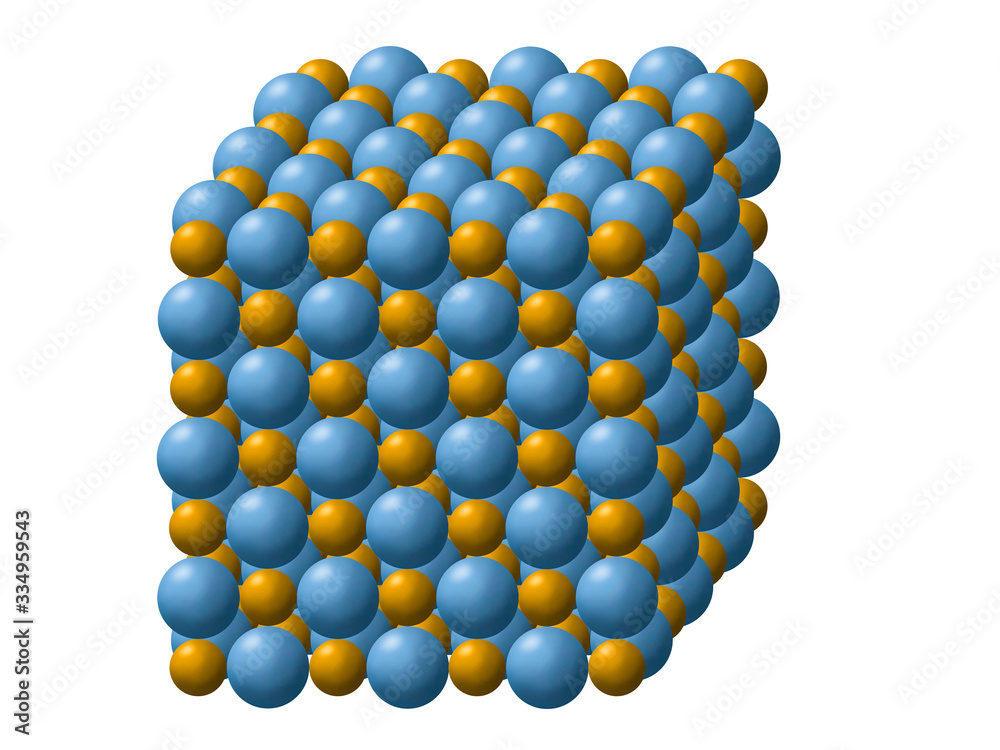
NaCl is interesting in that it is a three-dimensional checkerboard, and thus there are no NaCl molecules that exist in the structure.

This video shows the structure, where the atoms are located, how the NaCl structure can . CaC 2 (a salt-like carbide): Ca 2+ and linear C 22- .
Sodium Chloride (NaCl) Crystal
Beispiele für Vertreter dieses Strukturtyps sind neben Natriumchlorid selbst Lithiumchlorid (LiCl) und Kaliumchlorid (KCl), aber auch die meisten Erdalkalioxide (z. Less than that (down to 41%) then you get 6:6-coordination.Le chlorure de sodium est un composé chimique ionique de formule NaCl. Cl1- is bonded in a body-centered cubic geometry to eight equivalent Na1+ atoms.Everything you ever wanted to know about the NaCl crystal structure.
Structure of NaCl (Sodium chloride)
Layered Atom Arrangements in Complex Materials, A Technical Report from Los Alamos National Laboratory, Los Alamos, New Mexico, LA-14205, April 2006.Natriumchlorid-Struktur. des Zinnsulfidtyps stellen eine verzerrte Kochsalzstruktur dar. The scanning electron microscope (SEM) was used for surface morphology.NaCl is Halite, Rock Salt structured and crystallizes in the cubic Fm-3m space group.One of the simplest and most thoroughly studied ionic crystals is sodium chloride, also known as table salt.structure cristalline type NaCl.407 Å Z=4; Motif périodique: FeS 2 (type AX) S 2 2-est un ion double: Structure de la pyrite de fer : FeS 2: Positions atomiques Fe 2+ 0 , 0 , 0 S 2 2- ½ , 0 , 0 : Dernière mise à jour : . Le sel est un cristal, auto ses atomes forment une structure périodique et symétrique. Cas particuliers de densité de NaCl Image de James St.) Calculate the density of NaH.Natriumchlorid (auch Kochsalz genannt) ist das Natriumsalz der Salzsäure mit der chemischen Formel NaCl – nicht zu verwechseln mit Natriumchlorit (NaClO 2), dem Natriumsalz der Chlorigen Säure.
- Nano Rollo Für Fenster : Dachfenster Rollo ️ für jeden Fenstertyp in Top-Qualität
- Nachtwölfe Heidesee | Putins Biker-Gang: Wer sind die russischen Nachtwölfe?
- Naissance Enfant En France : Natalité en France — Wikipédia
- Naomie Harris Partner , James Bond-Star Naomie Harris wurde von Superstar begrapscht
- Namen Titel Kaufen : Doktortitel kaufen: Wie man sich einen falschen Titel kauft
- Nachbarwände Auf Grundstücksgrenze
- Nach Probearbeiten Keine Rückmeldung
- Names For Black Girls : 100 Electrifying Names Meaning Black: From Popular to Rare
- Nach Sport Niedriger Blutdruck Hoher Puls
- Nail Whitener Stiftung Warentest