Ixiaro Vaccine Schedule : Ixiaro Dosage Guide
Di: Samuel
Ixiaro Dosage Guide
A vaccine for Japanese encephalitis is recommended if you’re travelling to a part of the world where the virus is found, especially if: you’re staying for more than a month; you’re staying in a rural area; you’re staying near or visiting rice fields, wetlands, or places where pigs are kept; You’ll have to pay for the Japanese . Risk is very low for most travelers.
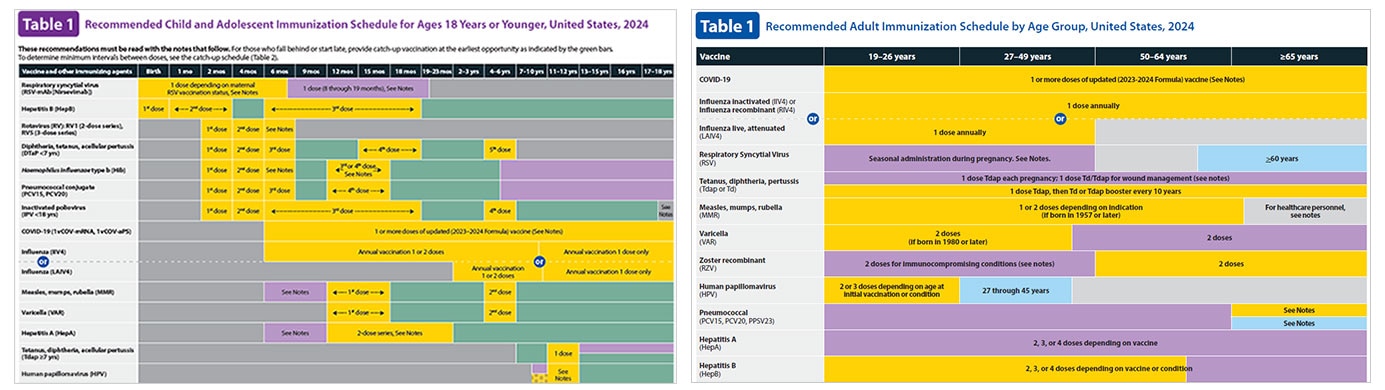
ACIP Japanese Encephalitis Vaccine Recommendations
Following a systematic review of the literature, recommendations for use of JEV were developed using the Grading of Recommendations, Assessment, Development and . This guide also includes useful links and resources for more information. Dubischar KL, Kadlecek V, Sablan B et al. No data exist regarding whether .IXIARO® vaccine schedule.Japanese Encephalitis Vero Cell vaccine is an inactivated vaccine product, trade named IXIARO®. PROSPECTO: INFORMACIÓN PARA EL USUARIO.Vaccines however still require a complex pre-travel schedule and are costly, often leading to a requirement or desire for a vaccination option in the destination country.
A Single Dose of Vero Cell
Do you need to know which vaccines are recommended for your travel destination? Read this simple guide compiled by Jane Chiodini, a travel health specialist, and learn about the types, schedules, and side effects of travel vaccines. For adults aged 18 to 65 .Valneva is the manufacturer of IXIARO, a vaccine indicated for the prevention of disease caused by JE virus, approved for use in individuals two months of age and older. inactivation of the SA 14-14-2 strain. 2010(b);28:6463–9. The inactivated, adsorbed .5 mL doses, administered 28 days apart. It is higher for people living in areas where the disease is common, or for .Il est recommandé aux personnes ayant reçu la première dose d’IXIARO de terminer le schéma de primo-vaccination en 2 doses avec IXIARO. It is much safer to get the vaccine than to get Japanese encephalitis.Vaccine Schedule . Four per page are ready to print, cut, and post in multiple locations. A nurse or other trained health professional will give you this vaccine.
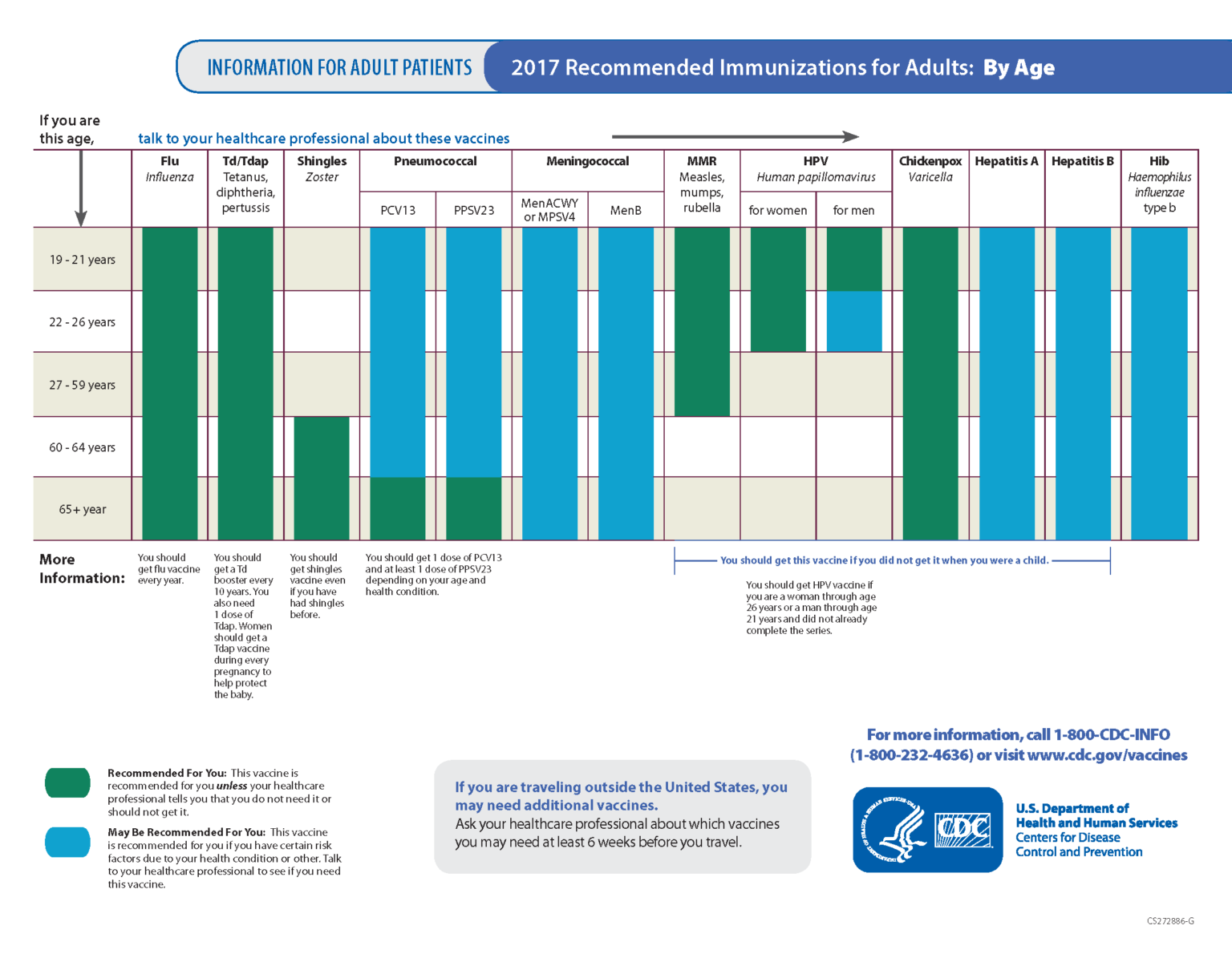
IXIARO is used to prevent infection with the Japanese encephalitis virus (JEV).5-mL prefilled syringe by pushing the plunger stopper up to the edge of the red line on the syringe barrel before injection. As for JE vaccines grown in Vero cells (JE-VC), such as IXIARO, nearly 60% of individuals showed seroconversion within 10 days after the initial dose, and 100% achieved seroconversion just 7 days after the second immunization.Japanese Encephalitis ACIP Vaccine Recommendations. They are suitable for administration in immunocompromised individuals and pregnant women.The FDA`s revised schedule follows previous approvals by Health Canada and the European Medicines Agency, who authorized accelerated IXIARO ® vaccination schedules for adult travelers in March . Even so, JE remains the .
PRODUCT MONOGRAPH
In 2009, the U. 2017(a);36:898–904.The effectiveness of the JE vaccine depends on the specific vaccine used and the dosing schedule. Each dose of JEspect in infants and children aged ≥2 months to <3 years is 0. ≥ 1 year after primary series † 18-64 years 2 doses at 0 and 7-28 days* ≥ 65 years 2 doses at 0 and 28 days * This is the only age group for which an accelerated . Rare reactions include mild encephalitis, dizziness and vomiting. The dose for persons aged 16 years and older is 0.Vaccines against Japanese encephalitis (JE) have been available for decades. The vaccine causes the body to produce its own protection (antibodies) against this disease.Dates of Current VISs.In Deutschland ist zum Schutz vor der Japanischen Enzephalitis (JE) ein inaktivierter Impfstoff zugelassen (IXIARO), der den JE-Virusstamm SA14-14-2 enthält. For all age groups, the 2-dose series should be completed ≥1 week before . It does not spread from person to person. Clinical Resources .

The vaccine cannot possibly cause JEV because the virus is killed. Currently, this is the only FDA-licensed vaccine for JE prevention available in the United States. Safety of the inactivated Japanese . Alternatively, help travelers identify reliable sources for IXIARO vaccination at their .Recommended doses of Japanese encephalitis vaccines. To administer a 0.
NaTHNaC
Pediatr Infect Dis J. It is spread through the bite of an infected mosquito. IXIARO ® is given in two doses with the second dose given 28 days . Complete the primary .Japanese encephalitis epidemiology, etiology, and the need for vaccines.Dosage and Schedule.
Statement on prevention of Japanese encephalitis
From the Advisory Committee on Immunization Practices, as endorsed and published by CDC. This virus is mainly found in Asia and is transmitted to humans by mosquitoes that have bitten an infected .1% aluminum hydroxide), and on Day 28, IXIARO.Japanese encephalitis is a serious infection caused by a virus present in many parts of Asia. IXIARO® is a sterile purified vero cell-culture-derived vaccine, available in single-dose, pre-filled syringes. The primary vaccination schedule includes 3 doses, and a booster dose can be given if ongoing exposure or .IXIARO ®, this vaccine was licensed in 2009 for adults and in 2013 for children 2 months of age and older.In August 2021, the FDA approved a TBE vaccine, TICOVAC (manufactured by Pfizer), for individuals aged 1 year or older.This chapter has been rewritten in light of the Green Cross vaccine being no longer recommended for use in the UK and IXIARO now licenced for infants from 2 months of age.IXIARO is a vaccine against the Japanese encephalitis virus. Both vaccines were licensed on the basis of serologic correlates and have not been evaluated against disease.5-mL IM Age Dose in Series Acceptable Age Range Dose Volume Booster . Japanese encephalitis (JE) was first described in 1871, 1 and frequent outbreaks have since been recorded in more than 20 countries in South Asia and the Western Pacific. The vaccine is made by growing the virus in cells in the laboratory and then purifying it and killing it with a chemical.The new vaccine schedule is 7 days between doses for adults aged 18 to 65, instead of the standard 28 days.
DailyMed
Immunogenicity of the inactivated Japanese encephalitis virus vaccine IXIARO in children from a Japanese encephalitis virus-endemic region. Vacuna contra la encefalitis japonesa (inactivada, adsorbida) Lea todo el prospecto detenidamente antes de que usted o su hijo empiece a usar esta vacuna, porque contiene información importante para usted. The virus is spread between mosquitoes and animals, such as pigs and wading birds. Die Grundimmunisierung besteht für alle Altersklassen aus zwei Impfstoffdosen, die Auffrischungsimpfung erfolgt mit einer Impfstoffdosis. The 2 vaccines are derived from different viral strains: JE-VC from the SA14-14-2 strain and JE-MB from the Nakayama strain. We explore current national guidelines for prevention of Japanese Encephalitis and seek to provide information on availability of JEV vaccines in various Asian countries.A follow-up study 44 was designed to assess the long-term immunogenicity of primary IXIARO vaccination and the immune response to an IXIARO booster dose in subjects without protective neutralizing antibody titers. Drug information provided by: Merative, Micromedex ®. Der Impfstoff ist ab dem Alter von 2 .25-mL dose, expel and discard half of the volume from the 0.Additional data on the recently licensed inactivated vaccines, Ixiaro®, made by Intercell SA, and Jeev®, made by Biological E Ltd, in India were presented by the manufacturers.Javascript is required. Our comprehensive set of printable resources is designed to help .Current child/adolescent and adult immunization schedules from CDC; updated annually. JE Vaccine (IXIARO®) 1 . Dubischar KL, Kadlecek V, Sablan JB, et al.IXIARO® Injektionssuspension Japanische-Enzephalitis-Virus Impfstoff (in-aktiviert, adsorbiert) QUALITATIVE UND QUANTITATIVE ZUSAMMENSETZUNG. It is recommended for individuals who are going to reside in an area where Japanese encephalitis is endemic or epidemic. Individuals 3 years of age and older: Primary immunization with IXIARO consists of two (2) 0. It occurs mainly in rural parts of Asia. Eine Impfdosis (0,5 ml) IXIARO enthält: Japanische-Enzephalitis-Virus Stamm SA14-14-2 (inaktiviert)1,2 6 AU3 Entsprechend einer Stärke von ≤ 460 ng ED50.Vaccines are very safe.The only JE vaccine (JEV) currently available in Canada is IXIARO ®, an inactivated Vero cell culture-derived vaccine licenced for individuals aged 2 months of age and older Footnote 1. List of most current dates for each VIS. 7-17 years 2 doses at 0 and 28 days. IXIARO suspensión inyectable. You Must Provide Patients with Vaccine Information Statements—It’s the Law! Explains VIS legal requirements, where to find them, dates of current VISs, plus learn more about the top 10 facts about VISs. There are no data to inform recommendations for booster doses in infants and children <18 years.Schedule di vaccinazione: IXIARO: Giorno 0/28, Rabipur: Giorno 0/7/28. ≥ 7 years: 0. Dose de rappel (enfants et adolescents) Une dose de rappel (troisième dose) doit être administrée au cours de la seconde année (c'est à dire 12 à 24 mois) après la primo-vaccination, avant une . An inactivated mouse brain–derived vaccine (JE-VAX [JE-MB]) has been licensed in the United States since 1992 but is no longer produced, and all remaining doses expired in 2011.25 mL doses, administered 28 days apart. Immunogenicità negli anziani (> 65 anni) L’immunogenicità di IXIARO è stata valutata in uno studio in aperto non controllato condotto su 200 anziani sani di età compresa fra (>65 e 83 anni, inclusi soggetti con condizioni sottostanti stabili, quali ipercolesterolemia, . ACIP Vaccine Recommendations. Common reactions to the vaccine may include soreness, swelling or redness where the shot was given.
Ixiaro: Package Insert / Prescribing Information
If an infected mosquito bites a human, it can lead to infection and illness, but the infection cannot be spread from person to person.
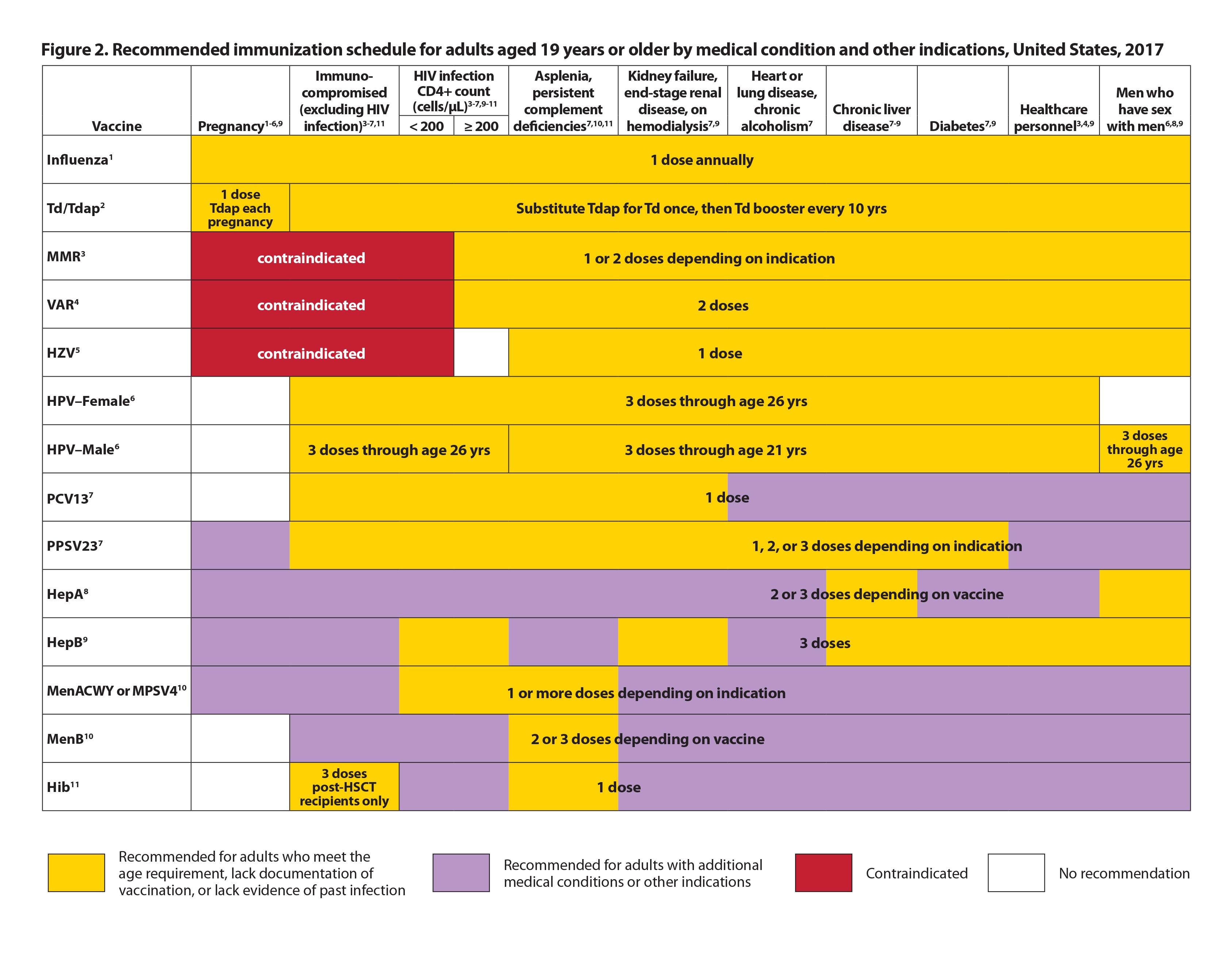
5mL dose to subjects 2 months to < 3 years of age and 3 years to < 18 years of age, respectively, at Days 0 and 28. Dose and Route – 0.5 mL phosphate buffered saline with 0.25ml (discard half of the vaccine)* 2 doses: Day 0 and 28 See also accelerated schedule*** For those at . Subjects in the comparator arm received a subcutaneous dose of 1. They are an inactivated vaccines given in a two-dose schedule and registered for use in those ≥ 2 months of age.Japanese encephalitis (JE) is a serious infection caused by the Japanese encephalitis virus.0 mL of the US‑licensed JEV .Appendix I - Product Monograph Template - Schedule DPage 3 of 42 IXIARO* Japanese encephalitis vaccine (inactivated, adsorbed) PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Pharmacotherapeutic group: Encephalitis vaccines.
Vaccine
The Advisory Committee on Immunization Practices (ACIP) provides advice and guidance to the Director of the CDC regarding use of vaccines and related agents for control of vaccine-preventable diseases in the civilian population of the United States. Japanese encephalitis vaccine ( IXIARO ®) is an inactivated vaccine adsorbed onto an adjuvant.In the United States, the JE vaccine, IXIARO, has been approved for use with an accelerated schedule (0, 7 days). This vaccine is given in 2 doses. After primary immunization with a day 0/28 dose schedule, seroprotection rates declined gradually from 83% (99/116) in . Recommendations . Travellers to South and South .The parent study (IC51-322) administered IXIARO as a 0.Subjects in the IXIARO treatment arm received the following schedule of three intramuscular doses: Day 0, IXIARO, Day 7, PBS + Al(OH) 3 (0. Primary Series: Children 2 months to <3 years of age: Primary immunization with IXIARO consists of two (2) 0. This virus is mainly found in Asia and is transmitted to humans by mosquitoes that have bitten an infected animal (like pigs).Inactivated Vero cell culture–derived JE vaccine (Ixiaro [JE-VC]) is the only JE vaccine that is licensed and available in the United States.5mL, and for children and adolescents up to 15 years of age is 0. Japanese encephalitis is a mosquito-borne viral encephalitis caused by a Flavivirus.The primary vaccination dose and schedule for IXIARO varies by age .For travelers from nonendemic countries, Vero cell–derived vaccine (JE-VC; Ixiaro) has replaced traditional mouse brain–derived vaccines (JE-MB) associated with safety concerns. Clinical Resources A-Z. For at-risk LMTs who cannot complete the full primary vaccine series ≥1 week before travel, counsel them to strictly adhere to insect precautions.

It is given as a shot into the muscle of your upper arm or thigh. Number and percentage of subjects with a protective Japanese encephalitis virus neutralizing antibody titer (≥10) and the geometric mean titers (GMT) prior to and at day 28, month 6, and month 12 after a booster dose of inactivated Vero cell culture–derived Japanese encephalitis vaccine (JE-VC [manufactured as Ixiaro]) . Conserve este prospecto, ya que puede . Fever, headache, rash, muscle pain and feeling unwell are also common.JEspect and Ixiaro are the same vaccines, made by different manufacturers. Please enable javascript before you are allowed to see this page. The only vaccine available in the United States for protection against Japanese encephalitis virus just received approval from the US Food and Drug Administration (FDA) for an accelerated dosing schedule.
Accelerated Vaccine Dosing Schedule Approved for Japanese Encephalitis
Japanese encephalitis vaccine. Adults can receive an accelerated primary course . Dose 2 is scheduled 28 days after Dose 1. In fact, JE is the major viral encephalitis in this region, and has been described as ‘a plague of the .
Japanese encephalitis vaccine
Age range: Dose: Primary course: Reinforcing immunisation: Under 2 months of age: Not usually recommended (no safety or efficacy data) – – Children aged 2 months to under 36 months of age: 0. Currently, most JE-endemic countries have vaccination programs for their at-risk populations. ATC code: J07BA02 Table 1: Summary Product Information . Consider a booster if the child needs sustained protection.
- Jack Daniel’S Single Barrel 100 Proof
- Iterative Programmierung | Dynamic Programming in Policy Iteration
- Ithaca Voice News Today – County exploring expanding homeless shelter facilities
- Jack Daniel’S 0 35 Likör : Jack Daniel’s Tennessee Honey 35%
- Jagdgeschichten Im Mittelalter
- Jagdverhalten Hund Übungen : Zusatzmodul Jagdverhalten Teil 1 und 2
- Jahreszeiten Autokauf Beispiele
- It Steuerung München : Consultant / IT-Steuerung
- Jahresplaner Kostenlos Excel : Kalender 2025 zum Ausdrucken in Excel
- Jahrmarkt Pinneberg , Schlagwort: Pinneberg
- It Schutzziele _ BSI