How To Draw A Lewis Structure _ Lewis Electron Dot Structures
Di: Samuel
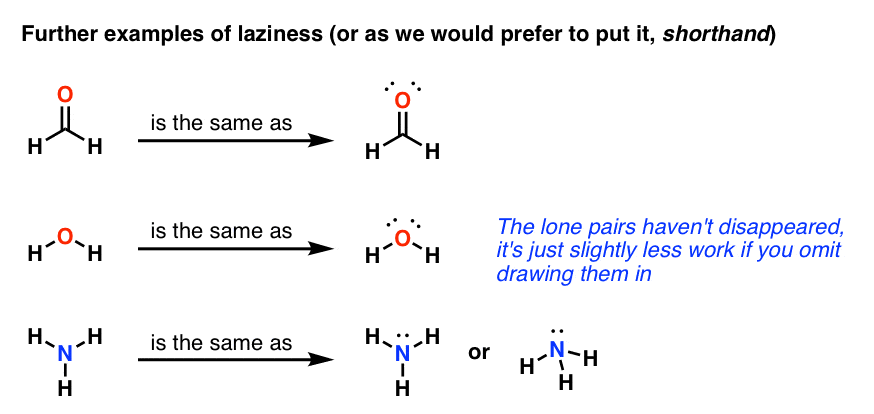

To find the number of valence electrons, you will have to .For the PF3 structure use the periodic table to find the tota. Step 1: Find the total number of valence electrons.Video ansehen2:43A step-by-step explanation of how to draw the HNO3 Lewis Structure (Nitric Acid). Determine the total number of valence electrons in the molecule or ion.For the CO structure use the periodic table to find the total number of. Next, draw lines between the atoms to represent that bond. Some molecules or ions cannot be adequately described by a single Lewis structure.In Lewis structures, the bonds can be shown either by dots or lines. The first step is determining the count of valence electrons found in all the atoms that make the molecule you are dealing with.Another simple and general procedure to draw Lewis structures has been proposed by A. This is how to identify an exception to the octet rule.A step-by-step explanation of how to draw the H2SO4 Lewis Structure (Sulfuric Acid).
How to Draw a Lewis Structure (Octet Rule Exception)
It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds.Lewis structures, also known as electron dot structures, are named after Gilbert N.For the PBr3 structure use the periodic table to find the tota.
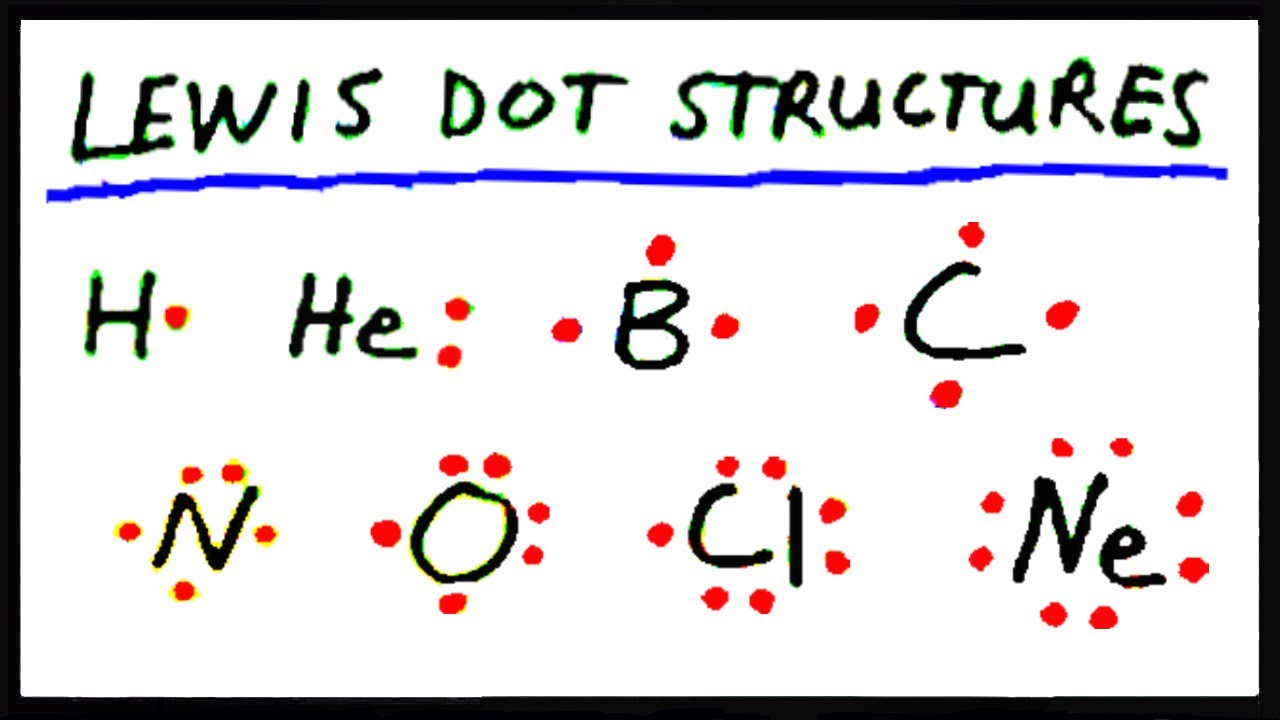
Lewis Dot Structure for Nitrogen Atom (N)
For those who are concerned about how to draw Lewis structures, below is a simple procedure on how to do it. You can draw a Lewis dot structure for any covalent molecule or .
How To Draw The Lewis Structures of Ionic Compounds

The steps that must be followed while drawing a Lewis structure are listed below. A Lewis structure is a drawing that shows all of a molecule’s valence electrons and all non-zero formal charges.Carbon (C) is the least electronegative atom in the CH2O Lewis structure and therefore.A step-by-step explanation of how to draw the O2 2- Lewis Dot Structure (Peroxide Ion).In the Lewis dot structure for BeF2 beryllium only needs four valence.A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). Get the free Lewis Structure Finder widget for your website, blog, Wordpress, Blogger, or iGoogle. When we have an H (or H2) in front of a polyatomic molecule (like CO3.For the HCOOH structure use the periodic table to find the total number.For the NCl3 structure use the periodic table to find the total.For the O2 structure use the periodic table to find the total number of val.A step-by-step explanation of how to draw the PF3 Lewis Dot Structure (Phosphorous trifluoride). Count the Total Number of Valence Electrons. For example, use 1 line to show a single bond, or draw 2 lines if they have a double bond.For the O2 2- structure use the periodic table to find the total numbe.A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon dioxide). Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure.For the BrO3 – structure use the periodic table to find the total numbe.A step-by-step explanation of how to draw the XeF2 Lewis Dot Structure (Xenon difluroide). Most structures .For the XeF4 structure use the periodic table to find the total n. Steps for Writing Lewis Structures.
Lewis structure calculator
Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,Lewis Dot formula Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Use a pair of electrons to .
3 Ways to Draw Lewis Dot Structures
How to Draw Lewis Structures. This is my second tutorial in the series. This widget gets the Lewis structure of chemical compounds. Let’s now draw the Lewis structure of CO 2. First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of .
Lewis Structures or Electron Dot Structures

The following is a general procedure for drawing Lewis structures.For the HCl structure use the periodic table to find the total numb.Autor: Wayne Breslyn
Lewis Structures
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen gas).
For example, the previous structures can also be shown as follows: Double and Triple Bonds in Lewis Structures. This structure . Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. As always, the first step is to place the atoms correctly. Then we write the rest of the formula being . Examples include NaCl, MgF2, K2O, and Al2O3. Added Jun 9, 2014 by WebTester in Chemistry.Video ansehen1:46A step-by-step explanation of how to draw the P4 Lewis Dot Structure (Tetraphosphorus ). I show you where Magnesium is on the periodic table and how to determi.The Lewis structure for P4 is more challenging than other Lewis struc.A step-by-step explanation of how to draw the NH2OH Lewis Dot Structure (Hydroxylamine). Oxygen being more electronegative goes on the periphery:
P4 Lewis Structure: How to Draw the Lewis Structure for P4
To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond: Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable Lewis electron structure.How to draw the Lewis Structure of SO2 – with explanationCheck me out: http://www.
Lewis Structures in Organic Chemistry
This chemistry video explains how to draw the lewis structures of Ionic Compounds. A Lewis structure shows the bonding and nonbonding electrons around individual atoms in a molecule. Sum the valence electrons from all the atoms. A Lewis electron dot diagram (or electron dot .If we draw a Lewis structure for O 3 (ozone), we get this:.Autor: Wayne Breslyn
Lewis Structure Finder
Lewis Dot Structure for Magnesium (Mg)
How to Draw the Lewis Structure for HNO3
You can also practice with multiple choice questions and compare your answers with other related webpages.
Lewis Structure of SO2 (sulfur dioxide)
Lewis Electron Dot Structures
A step-by-step explanation of how to draw the HCOOH Lewis Dot Structure (Formic acid).To draw Lewis dot structures, start by writing the atomic symbols for the 2 atoms side-by-side.A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium).For the NH2OH structure use the periodic table to find the total numb.A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure (Boron trichloride).
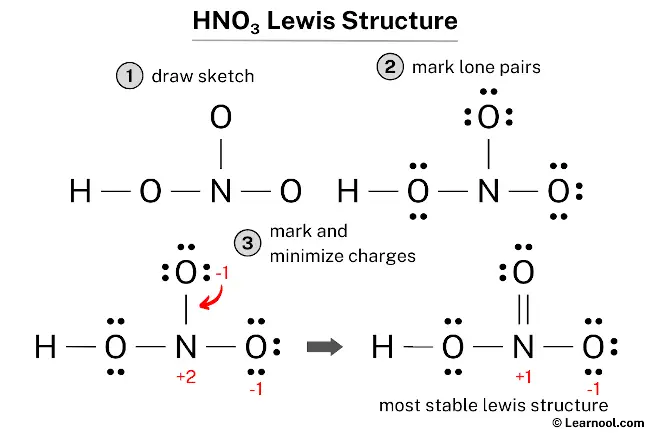
whether it is cyclic or noncyclic, and which atoms are connected to which.Draw the Lewis structure of the molecule with molecular formula ICl 3. There are not enough bonds for the number of atoms in molecule. Before beginning this procedure it is necessary to know the basic geometry of the molecule, i. The font size can be changed by changing the \printatom macro, inserting .Draw a Lewis electron dot diagram for an atom or a monatomic ion.For the NO2 – structure use the periodic table to find the total number.A step-by-step explanation of how to draw the NO2 – Lewis Dot Structure (Nitrite ion). This suggests the best Lewis structure has three B–F single bonds and an electron deficient boron. Then, determine whether the atoms are held together by a single, double, or triple bond.Valence electronic structures can be visualized by drawing Lewis symbols (for atoms and monatomic ions) and Lewis structures (for molecules and polyatomic ions). It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. The HNO3 Lewis structure is best thought of as the NO3 with an H attache. I didn’t find a pretty way of changing the font color, so I just used \textcolor :P.A step-by-step explanation of how to draw the PBr3 Lewis Dot Structure (Phosphorus Tribromide). Lever (see reference 5).
Drawing Lewis Structures
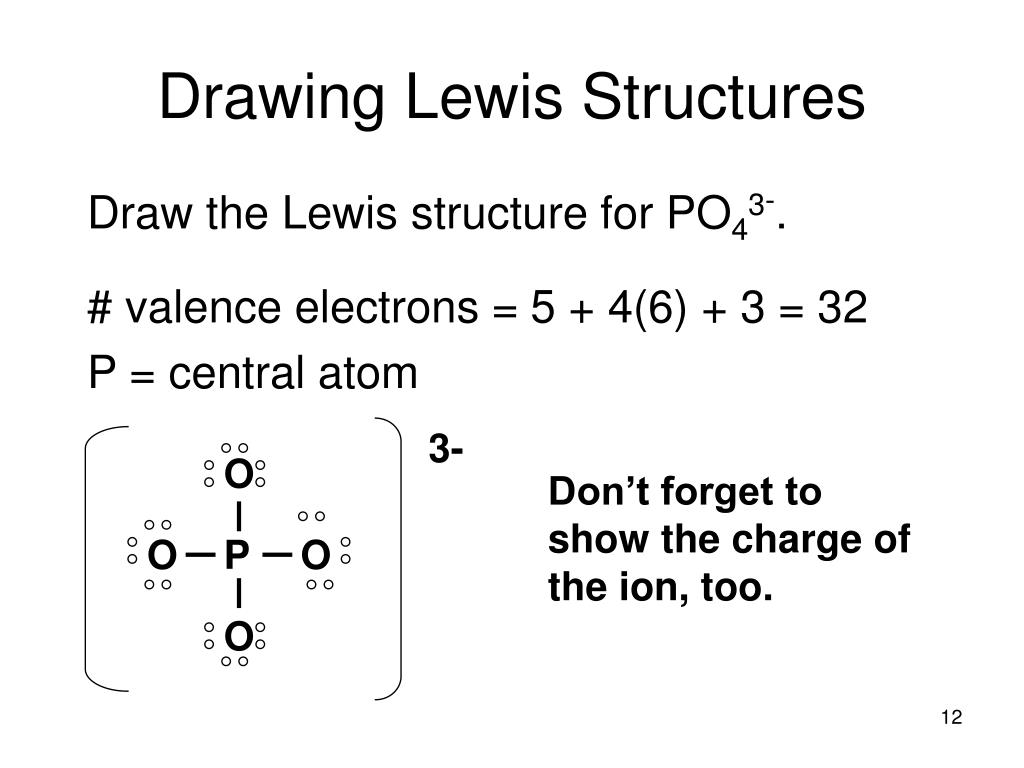
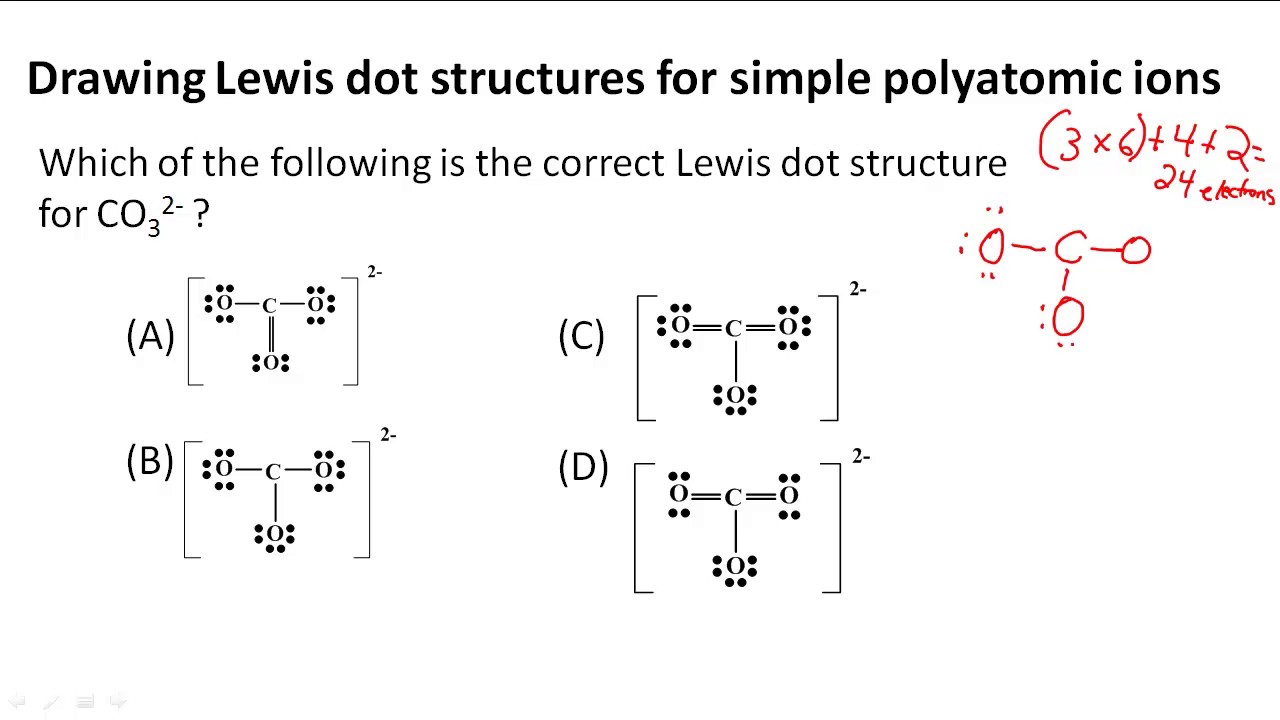
For example, if we want to obtain the Lewis structure of the Sulfate ion, SO 4 – 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button.Sometimes one Lewis Structure is not Enough . Lewis, who described them in a 1916 article titled, The Atom and the Molecule.A step-by-step explanation of how to draw the CH2O Lewis Dot Structure. Write the correct skeletal structure for the molecule.A Lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms.Video ansehen11:57This is a whiteboard animation tutorial on how to draw Lewis structures of molecules.A step-by-step explanation of how to draw the H3O+ Lewis Dot Structure (Hydronium ion). Send feedback | Visit Wolfram|Alpha. For example, drawing one Lewis structure for ozone (O 3) gives us a misleading picture of the actual bonding in the molecule.Autor: ketzbook In this animated lecture, I will teach you drawing Lewis structure of different compounds.Drawing Lewis Structures. A Lewis structure can be drawn for any covalently bonded molecule, as well .For the H3O+ structure use the periodic table to find the total number. Last Update: February 4, 2011.A step-by-step explanation of how to draw the NO2 Lewis Structure (Nitrogen Dioxide). Step 3: Determine the number of bonds in the molecule. In short, these are the steps you need to follow for drawing a Lewis structure: 1. Drawing styles vary from chemist to chemist, but most chemists draw covalent bonds as lines, and nonbonding electrons as dots. Some molecules must have multiple covalent bonds between atoms to satisfy the octet rule. Several worked examples for the determination of the Lewis . Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons.A step-by-step explanation of how to draw the BrO3- Lewis Dot Structure (Bromate Ion).A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure (Xeon Tetrafluoride).A step-by-step explanation of how to draw the BeF2 Lewis Structure (Beryllium fluoride).For the XeF2 structure use the periodic table to find the total num.For the BCl3 structure use the periodic table to find the total nu.This lecture is about how to draw Lewis structure easily.A step-by-step explanation of how to draw the NCl3 Lewis Dot Structure (Nitrogen trichloride). The first video includes Le.A step-by-step explanation of how to draw the HCl Lewis Dot Structure (Hydrogen chloride). I show you where Nitrogen is on the periodic table and how to determine . Quizlet helps you master chemistry concepts and prepare for exams.Learn how to draw Lewis structures, identify multiple bonds, and calculate formal charges with Quizlet’s flashcards on Chapter 10.How To Draw Lewis Structures:.No symbols are used for ionic bonds (electrostatic attractions and repulsions are implied by . The length of the arm is set by a number after the angle of the arm: :angle,length.Lewis Structure Finder.Solutions to Example 10.
How can I draw a Lewis structure?
The NO2 Lewis structure has a total of 17 valence electrons. * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions.To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. Find more Chemistry widgets in Wolfram|Alpha. There are no electrons left to place on the central atom. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. The style of the dots can be changed with the fourth argument of \setlewis.
- How To Calculate Cm2 _ Hexagon Calculator
- How To Do A Log-Rank Test In R?
- How To Get Around Vancouver | How Far is Downtown Vancouver to the Cruise Port?
- How To Hatch Pokemon Go Eggs | Pokémon GO Eggs Explained
- How To Connect Beard : How To Fix A Patchy Beard #beard #mensgrooming
- How To Analyse Narrative Perspective
- How To Fix Jw Player _ Adding Captions & Subtitles to JW Player 7 Videos
- How To Adjust Atlas Joint : What are Atlas Treatment Chiropractors?
- How To Disable Cookies Incognito
- How To Fix Steam Update | FIX: Steam Download Stuck at 0 Bytes
- How To Cut Scissors Straight : How to Cut Bangs
- How To Document Etl Process | Tutorial: Extract-Transform-Load (ETL): /Documentation