How Does Benzene Work | Nucleophilic Reactions of Benzene Derivatives
Di: Samuel
and filters the air completely every 30 minutes. You should memorize the structures and formulas shown in Figure 16. In addition, the pi bonds in benzene are significantly less reactive than ’normal‘ pi bonds, either isolated or conjugated.

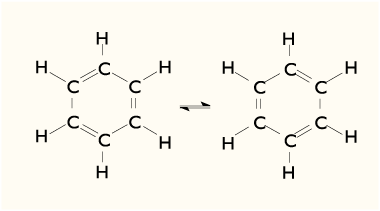
Benzene is a cyclic hydrocarbon (chemical formula: C 6 H 6), i.You can see how this works with the molecular orbital diagram for the aromatic compound, benzene, below.Benzene exposure can cause a number of health problems.0 license and was authored, remixed, and/or curated by LibreTexts. Breathing benzene can cause drowsiness, dizziness, and unconsciousness; long-term benzene exposure causes effects on the bone marrow and can cause anemia and leukemia. There are delocalized electrons above and below the plane of the ring.
Ozonolysis
7: Side-Chain Reactions of Benzene Derivatives is shared under a CC BY-NC-SA 4. Benzene has 6 \(\pi\) electrons. Most benzene exposure comes from the air from a number of sources, including forest fires, auto exhaust and gasoline from fueling stations.

Due to the unstable molozonide molecule, it continues further with the reaction and breaks apart to form a carbonyl and a carbonyl oxide molecule . As his part of the discussion is . Benzene, the commercial use of which dates to the late nineteenth century, was one of the earliest industrial chemicals demonstrated to affect the health of large numbers of workers [1,2]. For more information, call the ATSDR Information Center at 1-800-232-4636.The second factor that becomes important in reactions of substituted benzenes concerns the site at which electrophilic substitution occurs. In 1775, a British .William Reusch, Professor Emeritus ( Michigan State U. Both are derived from crude oil but have different applications and properties. Table 4 lists typical constant values. Your child’s doctor can determine the right . So How Does Benzene Compare? When we covered the reactions of alkenes a while back – a lot of reactions! – we saw that the vast majority fell into the class of reactions we call addition reactions. Lastly, Friedel-Crafts alkylation can undergo polyalkylation. Benzene has been found in at least 813 of the 1,430 National Priorities List .Benzene is one of the compounds used as octane enhancers in unleaded gasoline. Its first 2 \(\pi\) electrons fill the lowest energy orbital, and it has 4 \(\pi\) electrons remaining.Occupational exposure to benzene occurs through solvent exposures in the chemical industry, in petroleum .Warm benzene under reflux at 40°C with fuming sulfuric acid for 20 to 30 minutes. Mechanism Step 2: Pi electrons of benzene react with the acylium ion to form the sigma complex, resonance stabilized acylbenzenium intermediate: Mechanism Step 3: Deprotonation of the sigma comlex to restore aromaticity.
154 nm) C=C (0.Vent-free gas log sets operate by drawing in room air for combustion, and when airborne chemicals are present in the room, they can pass through the burner, resulting in a distinct odor reminiscent of petroleum-based products like kerosene. The molecular .7°C, which is considerably greater than the melting point of benzene (5. We know that benzene has a planar hexagonal structure in which all the carbon atoms are sp 2 hybridized, and all the carbon-carbon bonds are equal in length., each carbon atom in benzene is arranged in a six-membered ring and is bonded to only one hydrogen atom. Benzene, an aromatic hydrocarbon and a component of crude oil and gasoline, is produced at high levels and is widely used in the USA as an intermediate in the manufacture of plastics, resins, dyes, etc. In strong sunlight or with radical initiators benzene adds these halogens to give hexahalocyclohexanes.This places a positive charge next to the benzene ring, which is so strongly activating that the Friedel-Crafts reaction cannot occur. Alkenes Give “Addition” Products Upon Reaction With Electrophiles.Ortho hydrogens on a benzene ring couple at 6-10 Hz, while 4-bond coupling of up to 4 Hz is sometimes seen between meta hydrogens. The six carbon atoms form a perfectly regular hexagon. α-Naphthoic acid behaves similarly to . The electrophile is actually sulfur trioxide, SO 3, and you may find the equation for the sulfonation reaction written:
Leukemia and Benzene
This is divided into two or three doses per day. Ingestion of large amounts of benzene may cause vomiting, irritation in stomach, sleepiness, convulsions, rapid heart rate and death. This is the convention that will be used for the most part in this book.Reaction Mechanism.Grig nard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just ether).Safety Information.Although benzene is most often drawn with three double bonds and three single bonds, in fact all of the carbon-carbon bonds iare exactly the same length (138 pm).

Therefore a full reaction may look like this: Ozonolysis Reaction.

Benzene can cause blood cancers like leukemia.Short-term inhalation of high concentrations of benzene in air can cause headaches, dizziness, drowsiness, confusion and unconsciousness in humans.Benzene, C 6 H 6, is a planar molecule containing a ring of six carbon atoms, each with a hydrogen atom attached. It effectively removes heavy pungent odors (like benzene ), pet hair, dander, dust, fur, and germs. Side chain reactions can be used to create a wider range of aromatic compounds.The typical starting dosage is 0. However, this is not the case. Despite its prevalence, the cardiovascular effects of benzene have rarely been studied.Usually, derivatives of benzene (and phenyl groups, when the benzene ring is incorporated into a larger organic structure) are depicted with only one resonance contributor, and it is assumed that the reader understands that resonance hybridization is implied.Benzene is more susceptible to radical addition reactions than to electrophilic addition. tobacco smoke) known to increase cardiovascular disease (CVD) risk. Water will sublime from a solid (ice) to a gas (vapor) when the molecules have enough energy to break free . The fundamental principle in freeze-drying is sublimation, the shift from a solid directly into a gas. It was not until the 1930’s that Kekule’s structure was confirmed by X-ray and electron diffraction.
Do Air Purifiers Remove Benzene
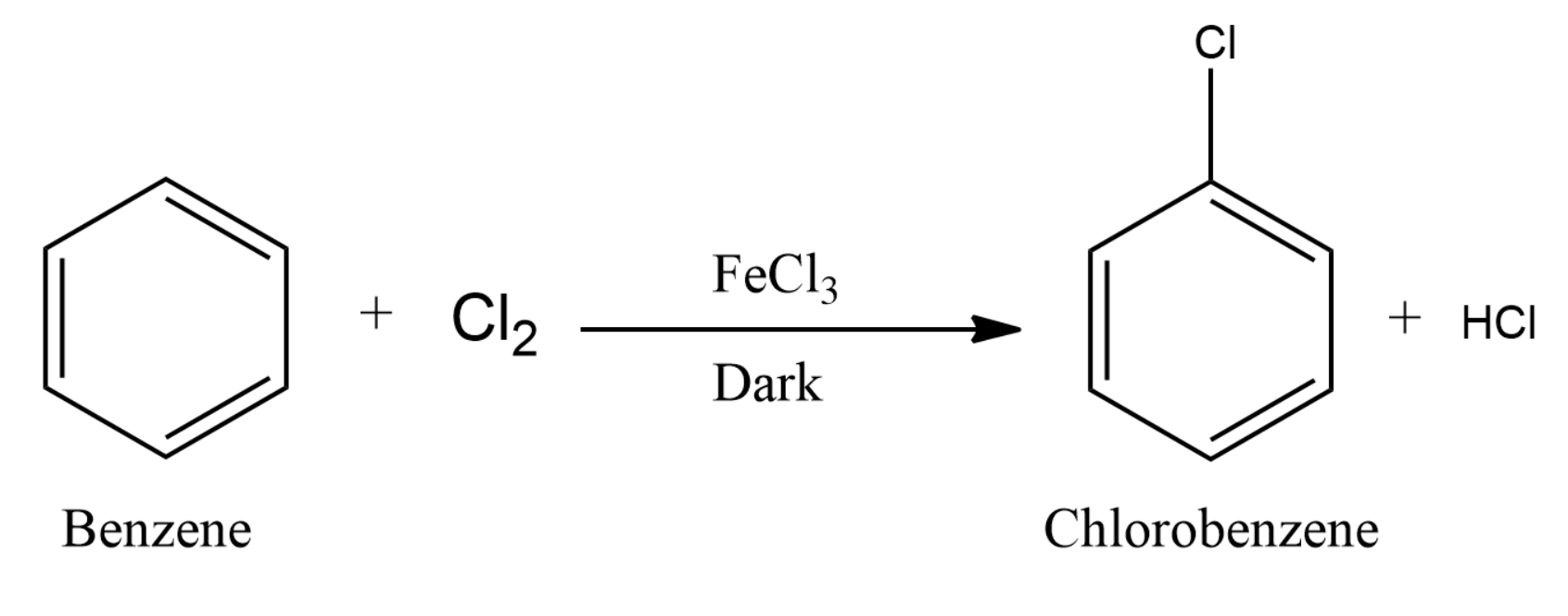
Many other substitution reactions of benzene have been observed, the five most useful are listed below (chlorination and bromination are the most common halogenation reactions). All of the carbon-carbon bonds have exactly the same lengths – somewhere between single and double bonds. The first scenario for adding an electrophile to a monosubstituted benzene ring is when the substituent is an electron donating group. ), Virtual Textbook of Organic Chemistry.Electron donating groups are alkyl groups, . The problem is that C-C single and double bonds are different lengths. Workers may be harmed from exposure to benzene. Protons directly attached to an aromatic ring, commonly called aryl protons, show up about 6. This method provides an opportunity to identify the reactive gas-phase intermediates during NTP reaction based on the following facts. Similarly, the scientific world’s attention should not be limited to them but should also extend its interest to non-workers residing in just adjacent industrial areas. Benzene is a colourless, volatile liquid with a characteristic sweet odour.
How do non-polar substances dissolve in non-polar solvents?
Benzene: general information
A phenyl group consists of a benzene ring with one of its hydrogens removed. 1: Two ways of representing a phenyl group.5C: Complex coupling.Benzene is a widely used chemical formed from both natural processes and human activities. Benzene can hinder the production of red blood cells, which will lead to anemia.Commonly seen is the use of O 3, which is ozone (structure shown above), in the presence of a reducing agent such as dimethyl sulfide (DMS, Me 2 S, S (CH 3) 2) or zinc & acetic acid (Zn/HOAc). In terms of chemical structure, benzene consists of a six .During the mid to late 1800’s, several possible structures (shown below) were proposed for benzene. It is described as an ‘aromatic’ hydrocarbon; each molecule of benzene is composed of a ring of 6 carbon .Benzene, a simple aromatic hydrocarbon with a sweet odor, is primarily used in chemical synthesis.
The Process
At higher temperatures, a more complex mixture of compounds is generated, including biphenyl, p -phenylbenzoic acid, and traces of benzaldehyde and 4,4′-dicarboxybiphenyl. The three general positions of a disubstituted benzene ring are ortho, meta and para. Since the reagents and conditions employed in these reactions are electrophilic, these reactions are commonly referred to as Electrophilic Aromatic Substitution.The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present. Very low levels of benzene have been detected in fruits, vegetables, nuts, dairy products, eggs and fish. The level of exposure depends upon the dose, duration, and work being done.Benzene is a planar molecule (all the atoms lie in one plane), and that would also be true of the Kekulé structure.A molecular orbital description of benzene provides a more satisfying and more general treatment of aromaticity. Fine (2-3 Hz) coupling is often seen between an aldehyde proton and a three-bond neighbor.Urinary benzene levels were linearly related to work shift air concentrations (N= 139, 4 samples per worker) over an exposure range of <0.That’s where we break a (relatively weak) C-C (pi) bond and form two .Characteristic 1 H NMR Absorptions of Aromatic Compounds. A hydrogen on the ring is replaced by a group like methyl or ethyl and so on. During the end of Kekule's career he revealed that the structure came to him in a vision after enjoying a glass or two of wine by the fire in his .Benzene also has a (weak but) permanent magnetic dipole due to its ring current, so some component of the attraction between benzene molecules may be magnetic and not coulombic. The most common long-term health effects, on the other hand, impact the blood.In our recent work, SVUV-PIMS was successfully introduced to an in-situ diagnosis of gaseous species evolution in the benzene decomposition reaction network in the NTP process [29]. The Alen BreatheSmart Classic eliminates it all using its 3-layer filtration method.Napalm-B, a napalm successor sometimes called super-napalm, NP2 or Incendergel, is made of 33 percent gasoline, 21 percent benzene and 46 percent polystyrene [sources: Browne, GlobalSecurity. The flask is fitted with a reflux condenser, and the mixture is warmed over a water bath for 20 - 30 minutes.

2–100ppm indicating that urinary benzene provides a biomarker of exposure over the previous 24 h, though the 95th confidence interval around the data extents over an order of magnitude [106–108]. Now as we said, instead of DMS under the arrow you may also see zinc and acetic acid. In other books or articles, you . Protons on carbons directly bonded to an aromatic ring, called benzylic protons, show up about 2.
Electrophilic Aromatic Substitution: The Six Key Reactions
For example, 1,4-dichlorobenzene, a compound used as an alternative to naphthalene in the production of mothballs, has a melting point of 52.134 nm) That would mean that the hexagon would be irregular if it had the Kekulé structure, with alternating shorter and longer sides . It is harmful to the eyes, skin, airway, nervous system, and lungs. Something about the structure of benzene makes its pi bonding . We have already noted that benzene does not react with chlorine or bromine in the absence of a catalyst and heat. According to molecular orbital theory, benzene ring involves the formation of three delocalized π – orbitals spanning all six carbon atoms, while the valence bond theory . The structure consists of 6 carbon atoms in a hexagonal ring, with alternating single and double carbon-carbon bonds. Benzene is treated with a chloroalkane (for example, chloromethane or chloroethane) in the .Water is added to isolate the acyl benzene final product.Benzene (C 6 H 6) is a highly flammable, colorless liquid that evaporates quickly into the air. 1: Each carbon in the benzene ring is sp2 hybrized with a p orbital perpendicular to the ring plane (Left) Being planar and cyclic allows benzene’s p orbitals to undergo cyclic overlap (Right) For this to happen, of course, the ring must be planar – otherwise the p orbitals couldn’t overlap properly and benzene is known .Benzene exposure has harmful health effects, especially in highly exposed population groups, including workers in certain industrial sectors. This problem does not occur during Friedel-Crafts . Gasoline, on the other hand, is a complex blend of hydrocarbons used as a fuel in vehicles. The mechanisms through which . Long-term inhalation exposure to lower levels of benzene .Exposure to benzene varied from low concentrations, thought to be without effect in humans, to high concentrations, ranging . Everything must be perfectly dry because Grignard reagents react .
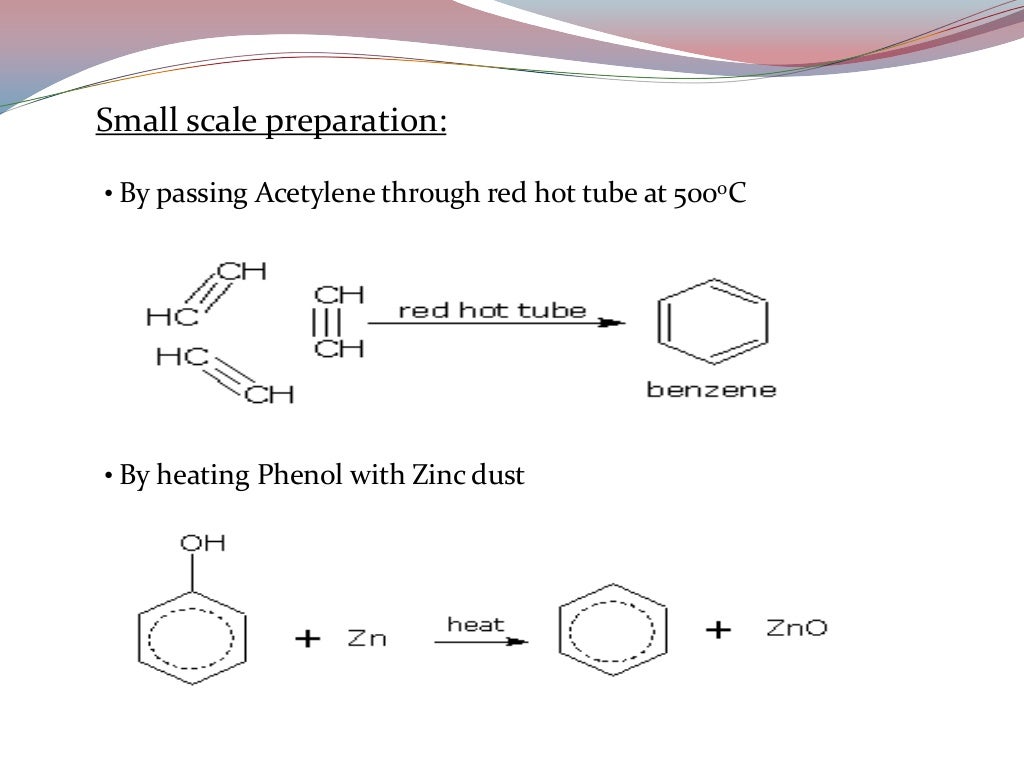
Benzene Factsheet
Or: The product is benzenesulfonic acid. Step 1: The first step in the mechanism of ozonolysis is the initial electrophilic addition of ozone to the Carbon-Carbon double bond, which then form the molozonide intermediate. It is manufactured by the catalytic conversion of acetylene to benzene: 3C2H2(. These 4 fill in the orbitals of the succeeding energy level. Just like evaporation, sublimation occurs when a molecule gains enough energy to break free from the molecules around it.Structure of Benzene. Certain aromatic hydrocarbons, such as benzene and benz[a]pyrene, are potent liver toxins and carcinogens. Since a mono-substituted benzene ring has two equivalent ortho-sites, two equivalent meta-sites and a unique para-site, three possible constitutional isomers may be formed in such a substitution. Or, the two attractions may be one and the same, as benzene’s electric quadrupole may result from its having a magnetic dipole. Short-term issues include dizziness, drowsiness, tremors, confusion, vomiting, and rapid or irregular heartbeat.
This range is typically called the aromatic region of an 1 H NMR spectrum. The reaction adds an electron donating alkyl group, which activates the benzene ring to further alkylation. Benzene also can be absorbed into the body by eating food or drinking water or other beverages contaminated with benzene. Mechanism Step 1: Acylium ion formation.Benzene gets into the air from forest fires, car emissions, gasoline vapors, and tobacco smoke. In all of the examples of spin-spin coupling that we have seen so far, . Figure 1: The Effect of an Electron Donating Groups on a Benzene Ring. This phenomenon occurs due to the nature of burning gas indoors without proper ventilation.
Nucleophilic Reactions of Benzene Derivatives
Alkylation means substituting an alkyl group into something – in this case into a benzene ring. It is worth noting that these same conditions . As shown below, the remaining cyclic array of six p-orbitals ( one on .The electrophilic substitution reaction between benzene and chloromethane.Benzoic acid decomposes around 500°C with the formation of benzene and CO2, following a typical decarboxylation reaction.
Benzene Class Action Lawsuit
The Alen BreatheSmart Classic is covers large areas up to 1100 sq.
Benzene in cigarette smoke is a major source of exposure. C6H6 +H2SO4 → C6H5SO3H +H2O (1) (1) C 6 H 6 + H 2 S O 4 → C 6 H 5 S O 3 H + H 2 O.03 milligrams per kilogram (mg/kg) of body weight per day. The gasoline in napalm is generally the same as that found at most gas stations, and that gasoline already has some benzene in it, but the benzene .A compound containing a benzene ring which has one or more alkyl substituents is called an arene. This public health statement tells you about benzene and the effects of exposure to it. The structure of benzene was determined many years ago, by a chemist called Kekule. People who work with petroleum products, including gasoline, are exposed to benzene by touching or breathing in the chemical. Hence, we examined whether exposure to benzene is associated with increased CVD risk. This suggests that benzene should react in the same way that an unsaturated alkene does. Notice how all of its bonding orbitals are filled, but none of the .Benzene is a ubiquitous, volatile pollutant present at high concentrations in toxins (e.
- How Do You Modulate The Colour Of An Electroluminescent Device?
- How Do You Write A Tv Show Pitch For Netflix?
- How Does Vpn Protect Anonymity
- How High Tech Fabric Cools _ Scientists create a fabric that cools the body
- How Do You Make An Electrolyte In A Lead Acid Battery?
- How Long To Take Paracetamol And Ibuprofen
- How Many Cities Are In Kerala?
- How Is Svn Different From Git – Subversion vs Git: The Pros and Cons
- How Hard Is It To Keep Up With Made With Unity Games?
- How Do You Play Bomb? – How To Make A Pipe Bomb
- How Do You Punctuate ‚And Of Course‘ In A Sentence?
- How Do You Name A Baby Clothes Brand?
- How Do You Cook Dumplings In A Frying Pan?
- How Is Hoarding Disorder Treated?
- How Do You Define A Mouth Shape?