How Do You Make An Electrolyte In A Lead Acid Battery?
Di: Samuel
After curing, let them dry for about 3-4 days. Now you are ready to fill your battery case with electrolytes made on your own.
Battery Reconditioning Epsom Salt
The volume of electrolyte governs battery capacity.
BU-201b: Gel Lead Acid Battery
The temperature of the newly prepared solution is very high, so it should not be injected into the lead-acid battery immediately. When the reaction is initiated, a current flows from the lead oxide . The acid is used as an electrolyte in the battery, and it is responsible for producing the electrical charge that powers the device. The acid-to-water ratio is usually between 1:4 and 2:3 (20-40% sulfuric acid) , depending on how much gravity you need. The process involves the following steps: Put on appropriate safety gear, such as gloves, goggles, and a lab coat, to protect yourself from the corrosive nature of sulfuric acid.A completely charged lead-acid battery is made up of a stack of alternating lead oxide electrodes, isolated from each other by layers of porous separators.
Lead-Acid Batteries: Chemical Management
This simple yet effective device measures the specific gravity of the electrolyte, providing insights into the battery’s health and charge level. If the gravity is at the desired level, you are done. For example, if the electrolyte .It is the first type of rechargeable battery ever created.44V (0% capacity).
Lead acid battery, Construction and, Working, and Charging
Formulation Of Electrolytes.In recent years, the market demand for lead-acid batteries is still growing []. A fuel cell requires an external supply of reactants as the products of the .Since this battery container primarily contains sulfuric acid as an electrolyte, the materials used to make a lead-acid battery container must be sulfuric acid-resistant.Electrolyte, in the context of lead-acid batteries, refers to a solution composed of sulfuric acid and water.At home, just put them in a pot that is filled with water into the oven and keep an eye on the water level.Making Electrolyte for the Lead Acid Battery: Add water to the Sulfuric acid; be very careful while making the electrolyte for the battery, after adding the water wait at least 30 minutes, so that the electrolyte can get cool. Intercell connectors connect the positive end of one cell to the negative end of the next cell hence the six cells are . Chemical reactions and the generation of electrical .The lead-acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté. Figure 1: Flow Battery. Hydrometers measure the specific gravity of the electrolyte to determine the state of charge. When we talk of battery acid, what comes to mind is mostly the electrolyte used in a lead-acid battery. Compared to modern rechargeable batteries, lead-acid batteries have relatively low energy density. Key Fact: The chemical reaction between the lead plates and the electrolyte generates electrical energy, powering the car’s electrical . It is made with lead electrodes immersed in a sulfuric acid electrolyte to store and release electrical energy. Finally, you can use the Hydrometer to check the specific gravity of the electrolyte. The lead-acid battery uses PbO 2 as the active material of the positive electrode and metallic Pb as the negative active material [3].It is recommended to check the electrolyte level at least once a month to ensure that the battery is functioning correctly.BU-201b: Gel Lead Acid Battery. You can quickly become frustrated, especially if you have an urgent appointment or meeting to attend.
Congratulations!
Here’s How You Can Make A New Lead Acid Battery Out Of Your
The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte.When adding battery water, you should never add tap water or bottled water. Too much water can also Dilute the sulfuric acid and cause the . While current flows through a metallic conductor in the form of lone electrons, within an electrolyte current flows in the form of . When charging, a buildup of positive ions forms at cathode/electrolyte interface.Figure 1 illustrates the flow battery concept.Each cell is made up of a set of positive and negative plates immersed in a dilute sulfuric acid solution known as electrolyte, and each cell has a voltage of around 2. The early gelled lead acid battery developed in the 1950s by Sonnenschein (Germany) became popular in the 1970s. Electrolyte is stored in tanks and pumped through the core to generate electricity; charging is the process in reverse. The two electrodes are separated by an electrolyte of sulfuric acid. The corroded plates will have a reduced ability to react and thus will significantly reduce the battery capacity. You might wake up one day, get into your car to start it, and discover it won’t start. Electrolytes for Rechargeable Batteries, Table 1 The properties of . Lastly, drain the acid into a container and do not discard it; we will need it later on.The 24V lead-acid battery state of charge voltage ranges from 25.7 kPa mmHg and a 23. As the battery charges, the sulfuric acid reacts with the lead in the anode and cathode to produce lead sulfate.These features, along .Stir the mixture well and Keep the mixture stored in the container for 8 hours to allow the acid to cool down.In principle, lead–acid rechargeable batteries are relatively simple energy storage devices based on the lead electrodes that operate in aqueous electrolytes with sulfuric acid, while the details of the charging and discharging processes are complex and pose a number of challenges to efforts to improve their performance.
Electrolytes for Rechargeable Batteries
Wait for the temperature to drop below 40℃, then measure the concentration of the solution and adjust it to the standard value, and then add it to the battery.
What Is Battery Acid? Sulfuric Acid Facts
Button batteries have a high output-to-mass ratio; lithium–iodine batteries consist of a solid electrolyte; the nickel–cadmium (NiCad) battery is rechargeable; and the lead–acid battery, which is also rechargeable, does not require the electrodes to be in separate compartments.BU-104b: Battery Building Blocks. Another way to tell is if the electrolyte levels are not equal in each cell.
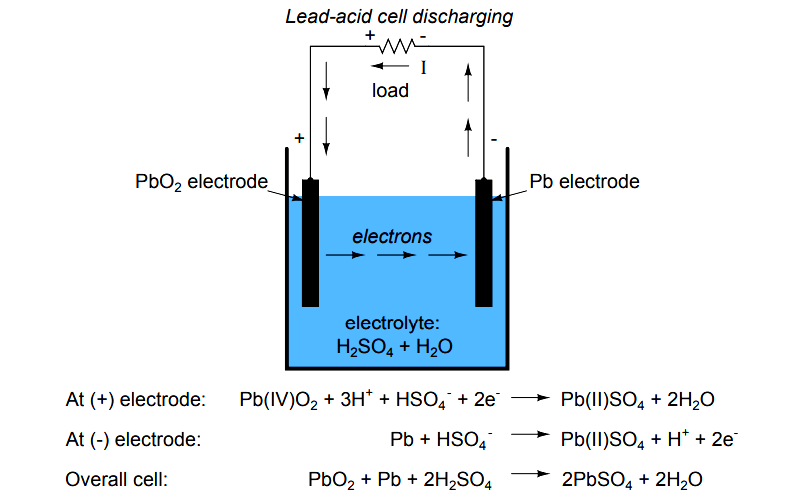
This electrolyte is a mixture of water and sulfuric acid in diluted form. And changes in temperature can alter our results. This process is called gassing, and it causes the electrolyte level to drop.The electrolyte in a battery is the substance that allows electrical current to flow between the anode and the cathode. This will reduce the battery capacity greatly. Tap water contains minerals that will react with the sulfuric acid in the battery.The ratio of distilled water and sulfuric acid in a battery is typically 1:1. In a 12-volt forklift battery, where the AH/6hr rating range is between 170 and 850 and the electrolyte content in gallons is between 3. Overall: Pb + PbO2 +2H2SO4 → 2PbSO4 + 2H2O.

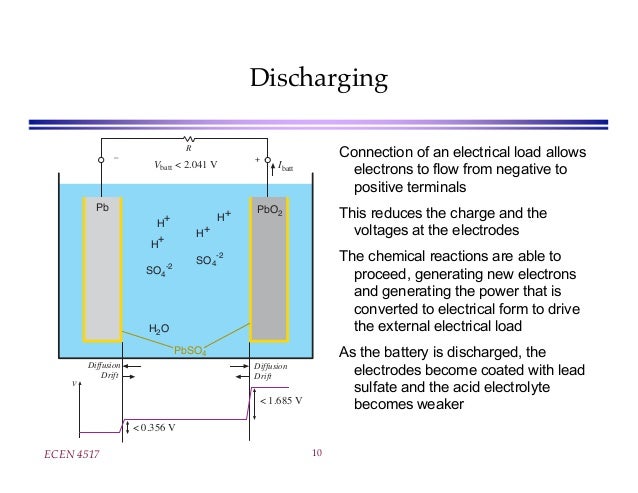
A reverse process occurs when the battery is discharging.Battery acid allows for the conversion of chemical energy to chemical energy. Battery acid is highly corrosive and able to cause severe burns.

This electrolyte is made up of sulfuric acid that is diluted in .by Bernard Ryan. Its manufacture and use continue to develop because of new applications for battery power in energy storage.The sulfuric acid content will vary depending on factors such as the voltage and the AH/6hr rating of the forklift battery.
Battery Acid Specific Gravity
When Does a Battery Need Electrolyte
Additionally, electrolyte consists of other components, such as metal salts and additives, which . Sulfuric acid is a mineral acid with the chemical formula H 2 SO 4. I will provide tips on how to check the electrolyte level and what to do if the level is too low or too high.Lead-acid batteries are successfully used in many applications [2]. Step 4: Adding Electrolyte. SLI batteries are designed to provide a burst of energy to start the engine and power the car’s electrical systems. Exercise care when pouring acid into the battery due to this chemical’s volatility.
A Guide to Water in Car Batteries
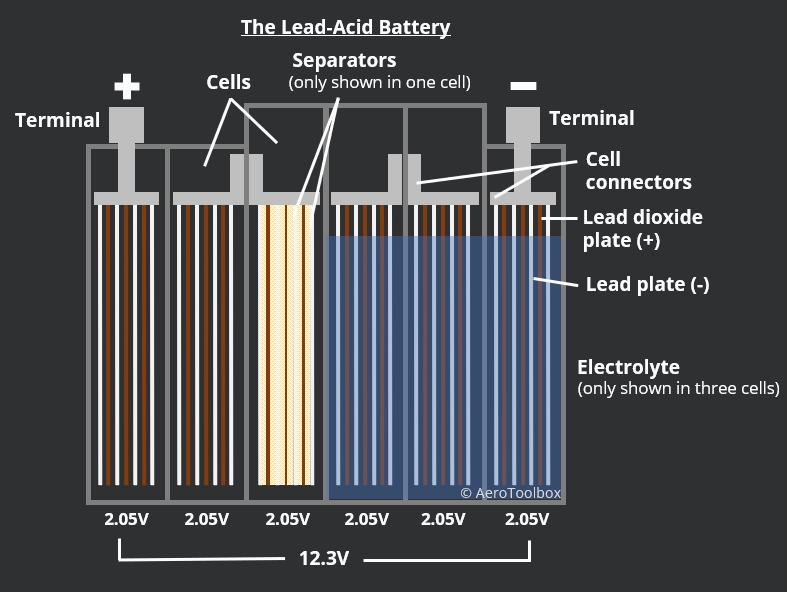
A lead-acid battery is a fundamental type of rechargeable battery. Use distilled water instead. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. Pb and PbO 2 are . The lead plates act as an anode and cathode, while the sulphuric acid is an electrolyte that contains hydrogen and . Marine and car batteries typically consist of multiple cells connected in series. At the cathode: PbO2 + 3H+ + HSO4– + 2e– → PbSO4 + 2H2O. Figure \(\PageIndex{3}\): One . The electrolyte loses much of its sulfuric acid content during this . Especially Iron and manganese are intolerable. The exposed lead plates will react with water in the atmosphere.When the battery acid levels are low, they will expose the battery plates.
How Do Lead Acid Batteries Work?
The 48V lead-acid battery state of charge voltage ranges from 50.Lead acid battery has a long history of development []. A rare occasion where you can go for more battery acid is if it spills.Voltammetry at rotating disc electrodes is used to define the conditions for the high rate deposition and dissolution of lead and lead dioxide in aqueous methanesulfonic acid.To make acid for a lead-acid battery, dissolve sulfuric acid in water.Battery acid is a common name for sulfuric acid (US) or sulphuric acid (UK). In this case, the battery acid is acidic with a pH of around 0. In a lead-acid battery, the negative and positive plates are dipped into a liquid formulation that is called electrolytes.Most vehicles use lead-acid batteries, known for their reliability and cost-effectiveness. This means that for every one part sulfuric acid, there is one part distilled water. An electrochemical battery consists of a cathode, an anode and electrolyte that act as a catalyst. Vanadium is the 23 rd element on the periodic table and is mined in China, Russia and South Africa.The electrolyte used in the battery may be liquid or solid and it may as well be acid or alkaline in nature. As the battery charges or discharges, the specific . This affects the density and specific gravity of the electrolyte.Batteries with KOH-based electrolytes can operate in a high-temperature range [ 6] due to the low vapor pressure of the solution, e. In this article, I will discuss how often you should check the electrolyte level in a sealed lead acid battery.
How to Make Acid for a Battery (4 Simple Steps)
In a lead-acid battery, the cathode is made of lead-dioxide, and the anode is made of metallic lead.

Using the hydrometer, check the gravity of the electrolyte.
Battery Acid Vs Distilled Water- The Primary Differences
What is Lead-Acid Battery?
Bring out your gloves and protective clothing.Lead acid batteries get their name due to the lead plates and sulphuric acid that are contained within them.The best way to tell if the battery needs more electrolyte is if the plates are exposed or coming close to exposure.
BatteryStuff Articles
The container material should also be free from those impurities which deterious to the sulfuric acid. Voltaic cells are also sometimes referred to as galvanic cells. You do not want to add another form of water and definitely not acid.When a lead acid battery is fully charged, the electrolyte is composed of a solution that consists of up to 40 percent sulfuric acid, with the remainder consisting of regular water.In a lead-acid battery, the anode is connected to lead plates on one side of the box, and the cathode is connected to lead dioxide plates on the opposite side. The battery acid is made up of sulfuric acid that is diluted with . AGM works best as a mid-range battery with capacities of 30 to 100Ah and is less suited for large systems, such as UPS.1 volts when fully charged. A standard solution is defined as “a solution that contains some number of grams of solute per liter of solvent.
Lead Acid Battery Voltage Chart: The Voltage Level Differences
The two lead plates are set opposite each other in the sulphuric acid and separated by an insulating material.
What is an electrolyte?
I’ve briefly introduced sulfuric acid and battery acid, their danger, and how to protect yourself, explained how to make it step-by-step, and .0 kPa at ambient temperature [ 7 ]. The reason for this is because sulfuric acid is very corrosive and can damage the battery if there is too much of it. When a lead-acid battery is charged, the electrolyte solution (a mixture of water and sulfuric acid) breaks down into hydrogen and oxygen gas, which escape through the vent caps.92 (100% capacity) to 45. When this reaction takes place, it will create sulfur compounds that do not break down when the charge current is introduced. Another example is the deep cycle battery, which is commonly used in marine . Lead-acid batteries have been in use for over a century and remain one of the most widely used types of batteries due to their reliability, low cost, and relatively .Electrolytes may be fluids or solids.The main components of lead-acid batteries are lead and/or lead oxide and the electrolyte (sulfuric acid and water).To create a lead-acid battery electrolyte solution, you will need to mix sulfuric acid (H 2 SO 4) with distilled water.The optimal time to add water to a lead-acid battery is during its charging cycle. A battery hydrometer is an indispensable tool for anyone involved in battery maintenance, especially for lead-acid batteries. The six cells .72V (0% capacity).The electrolyte solution in a lead-acid battery expands when warm and contracts when cold. Most people often think buying a new battery .Despite this, they are able to supply high surge currents.As an Amazon Associate we earn from qualifying purchases made on our website. First off, cut the top off the battery and leave about .
How Much Sulfuric Acid Is In A Forklift Battery?
Mixing sulfuric acid with a silica-gelling agent converts liquid electrolyte into a semi-stiff paste to make the gel maintenance free. It is a solution of sulfuric acid and water , with a concentration that can range from 20% to 50%. In this case, electrolyte simply means distilled water. Both the liquids are mixed together in 5:3 formation to make the acid less volatile.1 wt % solution about 2.And constantly stir the solution.Other components should be reviewed as well; however, neither antimony or polypropylene are listed in Appendix A and B, so the general threshold of 10,000 pounds would apply to them if you’re reporting by component (unless . If there is not enough acid, you do not fill it with more acid, as it is hazardous. Measure the required amount of distilled water . In lead-acid batteries, the concentration of sulfuric acid in water ranges from 29% to 32% or between 4.The lead–acid battery is a common battery used to provide the starting power in virtually every automobile and marine engine on the market.The AGM suspends the electrolyte in a specially designed glass mat. This offers several advantages to lead acid systems, including faster charging and instant high load currents on demand. This leads electrons moving towards the cathode, creating a voltage potential between the cathode and the anode. The middle is made up of alternating lead and lead dioxide plates surrounded by sulfuric acid (the electrolyte).
How to Use a Battery Hydrometer: Avoid 6 Common Mistakes!
This solution serves as a medium for the chemical reactions that occur during the battery’s charging and discharging cycles. The AGM that arrived in the early 1980s offers similar performance . The total voltage generated by the battery is the potential per cell (E° cell) times the number of cells.This series of papers will describe the chemistry, electrochemistry and performance of a flow battery with no separator and a single electrolyte, lead(II) in methanesulfonic acid.Batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity.One common example of lead-acid batteries is the starting, lighting, and ignition (SLI) battery, which is commonly used in automobiles. All these parts are placed in a concentrated solution of sulfuric acid.Battery acid is a highly corrosive liquid found in lead-acid batteries. Soluble salts, acids, and bases can generally act as electrolytes. These batteries consist of lead plates submerged in an electrolyte solution, typically a mixture of water and sulfuric acid.5 wt % solution shows a vapor pressure of about 0. It is important to note that the voltage range for your specific battery may differ from the values provided in the . When you recharge your .A battery acid specific gravity is defined as “the ratio of the density of the battery acid, relative to water with which it would combine if mixed evenly”.Through continuous development and technological progress, lead-acid batteries are mature in technology, safe in use, low in cost, and simple in maintenance, and have been widely used in automobiles, power . The following half-cell reactions take place inside the cell during discharge: At the anode: Pb + HSO4– → PbSO4 + H+ + 2e–.
Lead-Acid Batteries: Examples and Uses
46V (100% capacity) to 22. As the battery discharges, the positive and negative plates gradually turn into lead sulfate. This reaction with water causes the plates to corrode.08, the sulfuric acid content in gallons is typically between 0.
- How Do You Say Good Day In Albanian?
- How Do I Turn Off Pop Up Ads On My Computer?
- How Do You Play Bomb? – How To Make A Pipe Bomb
- How Far Can The New Astra Electric Go?
- How Do U Spell Ax : Cursive Alphabet [Letters A to Z, Worksheets and Tutorials]
- How Do I Update The Audio Driver On Windows 10?
- How Is Hoarding Disorder Treated?
- How Do Ski Touring Poles Work?
- How Effective Is Plan B – How effective Plan B is depends on how soon you take it after sex
- How Do You Beat Level 15 On 40X On Escape?
- How Do You Treat A Piercing Infection?