How Do You Calculate Half Life If A Quantity Decays Exponentially?
Di: Samuel
1: The half-life of iodine-131 is eight days. k = rate of growth (when >0) or decay (when <0) t = time.
Radioactive half-life
It is given by DXc]. Note: This is the same expression we came up with for doubling time. The half-life formula is commonly used in nuclear physics where it describes the speed at which an atom undergoes radioactive decay.
Drug Half-Life Calculator
The symbol for half-life is t½.The half-life is different for different nucleoids, as shown in Fig. If we replace this in equation 3, we obtain: N02=N0e-t1/2. M = the ending amount. Here is the first equation: (1/2) number of half-lives = decimal amount remaining. In 14 more days, half of that remaining half will decay .Doing half-life problems is focused on using several equations.To calculate the half-life, we want to know when the quantity reaches half its original size.The half-life of a drug is an estimate of the time it takes for the concentration or amount in the body of that drug to be reduced by exactly one-half (50%).
Half-Life Calculator
Q = (mRa − mRn − mHe)c2.
Study Guide
If you are given a problem where you are told how many half-lives have elapsed as well as how much time has passed, you .Growth and Decay. The farther a nucleoid is away from the stable nucleoid (shown by black dots in Fig. The calculator will set the unit of the result automatically.27 years (Figure 11.The second half-life has an activity of half the previous count (not the initial count). How to calculate half life? To find half-life: Find the substance’s decay constant. y′ = −ky0e−kt = −ky. [Math Processing Error] y 0 2 = y 0 e − k t 1 2 = e − k t − ln 2 = − k t t = ln 2 k. In a given cobalt-60 source, since half of the Co2760 Co 27 .or combining equations 1B and 11. To find N N, we first find the number of 12C 12 C nuclei in 1. n = 3 = 30days 10days n = 3 = 30 d a y s 10 d a y s. Let us use several different half-lives to illustrate this equation. It varies from a fraction of a second to more than 10 20 s, i. (4) Solving this equation for t1/2 yields: t1/2=ln (2) (5) This means we can determine an element’s half life by measuring its decay constant from experimental data. Dosage – the amount of the drug administered at .Radioactive decay law: N = N. a = value at the start. This time is called the doubling time. Then we do a little bit of math to get the decay constant.Carbon-14 nuclei are produced when high-energy solar radiation strikes 14 N 14 N nuclei in the upper atmosphere and subsequently decay with a half-life of 5730 years.Since the number of electrons on each side of the reaction is equal, you can use atomic masses to determine Q.K-40 decays by positron emission and electron capture to form Ar-40 with a half-life of 1. If you receive a sample of pure 83Sr and must complete a study of the nuclide before 3.e it refers to the time that a particular quantity requires to reduce its initial value to half. Therefore, we have.Every radioactive isotope has a half-life, and the process describing the exponential decay of an isotope is called . The sample’s age is the time elapsed rounded off according to the standard convention mentioned in Stuiver and Polach, 1977. Applications of the Half-life Formula. Other methods, such as rubidium-strontium dating (Rb-87 decays into Sr-87 with a half-life of 48.
ChemTeam: Half-Life
To determine the number of half-lives (n), both time units must be the same., 690 (+/-5) years, as the final result.60 kg of U-234, how long would it take for the .Half-life (t½) is the amount of time required for a quantity to fall to half its value as measured at the beginning of the time period. We can use the formula.View full document. This field is already prefilled. In your case, iodine-131 is said to have a .5)number of half-lives N t = N 0 × ( 0. If you don’t know the half-life of the drug, take a look at our table below. = the beginning amount.Carbon-14 has a half-life of 5,730 ± 40 years—i.025402u − 222. For example, if 100mg of a drug with a half-life of 60 minutes is taken, the following is estimated: 60 minutes after administration, 50mg remains.
Nuclear Half-Life Calculations
The rate of nuclear decay is also measured in terms of half-lives.Based on the last equation, half life is the value of t for which N=N0/2. The quantity after time t is the original quantity times this factor of left parenthesis one half right parenthesis Superscript t divided by Upper . The half time of a given drug – this input can be either in minutes, hours, or days. It is given by.00% of the material decays, how long do you have to complete the required study . But sometimes things can grow (or the opposite: decay) exponentially, at least for a while., half the amount of the radioisotope present at any given time will undergo spontaneous disintegration during the succeeding 5,730 years. Half-life = ln 2 k .The model is nearly the same, except there is a negative sign in the exponent.8: Exponential Growth and Decay is shared under a CC BY-NC-SA 4. You can’t tell when a green will turn into a red, or which green will decay, but after every half-life, half of the green parents will have decayed into red daughters . In other words, y‘ = ky.In order to use our 1/2 life calculator you’ll need the following data:.As time (t) increases, this factor approaches 0, indicating an exponentially decaying quantity due to radioactive decay. Write the complete .
Exponential decay problem solving (video)
y = y 0 e − k t. The half-life of the sample is (A) 5 min (B) 7.693N t1/2 R = 0.There is a substantial number of processes for which you can use this exponential growth calculator.In most problems, the half-life of a radioactive element is given to you. It is given by \((\ln 2)/k\) It is given by \((\ln 2)/k\) This page titled 6. In this question (t½) is 45 minutes , which means that after 45 minutes half of the sample would have decayed and half would be left as it is. DED A N- Trust NI Lett be the amount of time that has passed.JEE Main 2022: A radioactive sample decays (7/4) times its original quantity in 15 minutes. Determine the decay rate of Carbon-14. Equation 11 is a constant, meaning the half-life of radioactive decay is constant. One example of how to use the equation: One of the Nuclides in spent nuclear fuel is U-234, an alpha emitter with a half-life of 2. Listed below (see table below) are the half-lives of some common and important radioisotopes. For example, the half-life of carbon-14 is 5,730 years. For example, cobalt-60, an isotope that emits gamma rays used to treat cancer, has a half-life of 5. You can find a half-life calculator online to simplify the process of solving half-life problems.Example 1 – Carbon-14 has a half-life of 5. Half of a given sample of iodine-131 decays after each eight-day time period elapses.One of the common terms associated with exponential decay, as stated above, is half-life, the length of time it takes an exponentially decaying quantity to decrease to half its original amount.46 Radiocarbon Dating One of the most common applications of an exponential decay model is carbon dating. From population growth and continuously compounded interest to radioactive decay and Newton’s law of cooling, exponential functions are ubiquitous in nature. y 0 2 = y 0 e − k t 1 2 = e − k t − ln 2 = − k t t = ln 2 k. This is a favorite isotope in physics labs, since it has a short half-life and decays to a stable nuclide.
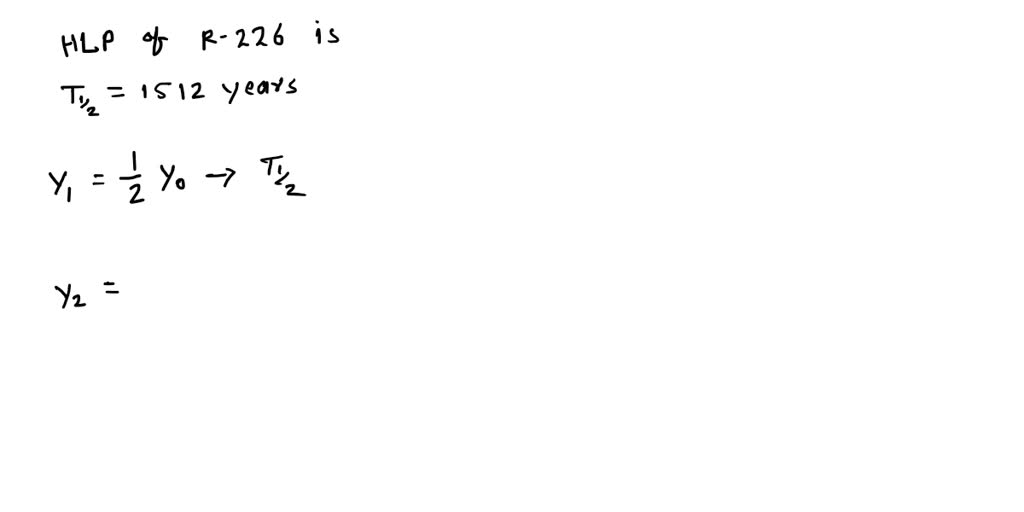
If a rock sample is crushed and the amount of Ar-40 gas that escapes is measured, determination of the Ar-40:K-40 ratio yields the age of the rock.c`]\ >ce 3 L / Example 6. Where y (t) = value at time t. The decay constant, λ, which is the same as a rate constant discussed in the kinetics chapter., more than 3 trillion years. Radioactive carbon has the same chemistry as stable carbon, so it combines with the ecosphere and eventually becomes part of every living organism. 720 hours × 1day 24hours = 30days 720 h o u r s × 1 d a y 24 h o u r s = 30 d a y s. The quantity after time t is the original quantity times this factor of t () OB. The general rule of thumb is that the exponential growth formula: x (t) = x_0 \cdot \left (1 + \frac {r} {100}\right)^t x(t) = x0 ⋅ (1 + 100r)t. Use the exponential decay formula to calculate k, calculating the mass of carbon-14 remaining after a given time, and calculating the time it takes to have a specific mass remaining. This is a hypothetical radioactive decay graph.1), the less stable it is, and the faster it decays.
Radiocarbon Dating Calculator
Exponential decay refers to a process in which a quantity decreases over time, with the rate of decrease becoming proportionally smaller as the quantity gets smaller. The order in which you use them depends on the data given and what is being asked. The formula is \(t_{\frac{1}{2}}\)= 0.5 ( t / T) In this equation: N (t) refers to the quantity of a radioactive element that exists after time t has elapsed.Half life formula.
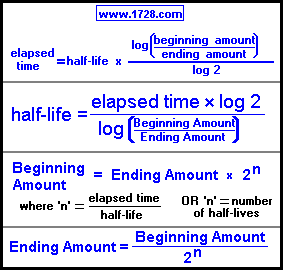
Here are a few uses for the half-life formula. Systems that exhibit exponential growth follow a model of the form y = y0ekt.Exponential growth and decay show up in a host of natural applications.44 x10^5 years.

Half-lives have a very wide range, from billions of years to fractions of a second. The spontaneous breakdown of an atomic nucleus of a radioactive substance causing the emission of radiation from the nucleus is known as Radioactive .

To calculate the half-life, we want to know when the quantity reaches half its original size.The half-life is the time it takes for half of a given amount of an isotope to decay. Here you will learn: What is the half-life in exponential decays; How to calculate the exponential . In other words, it takes the same amount of time for a population of bacteria to grow from \(100\) to \(200\) bacteria as it does to grow from \(10,000\) to \(20,000\) bacteria. The half-life is the time it takes for a given isotope to lose half of its radioactivity. However, when the organism dies, the amount will decrease over time.Half-life refers to the amount of time it takes for half of a particular sample to react i.Calculate the half-life of a radioactive substance that decays by a factor of 3 in concentration every 1 million years.Question: Given a half-life, explain how you calculate the value of an exponentially decaying quantity at any time t GHED Choose the correct answer below OA Lett be the amount of time that has passed. As with exponential growth, there is a differential equation associated with exponential decay. Exponential growth and exponential decay are two of the most common applications of exponential functions. Given a half-life, explain how you calculate the value of an exponentially decaying quantity at any time t.

Exponential Growth Calculator
If a quantity decays exponentially, the half-life is the amount of time it takes the quantity to be reduced by half. Solution – If 100 mg of carbon-14 has a half-life of 5. In essence, the half-life tells you at what time intervals you can expect an initial sample of a radioactive isotope to be halved. Half-life and the radioactive decay rate constant λ are inversely proportional which means the shorter the half-life, the larger λ and the faster the decay.1 can be used to calculate the amount of radioactivity remaining after a given time: Nt = N0 ×(0.
Radioactive Decay
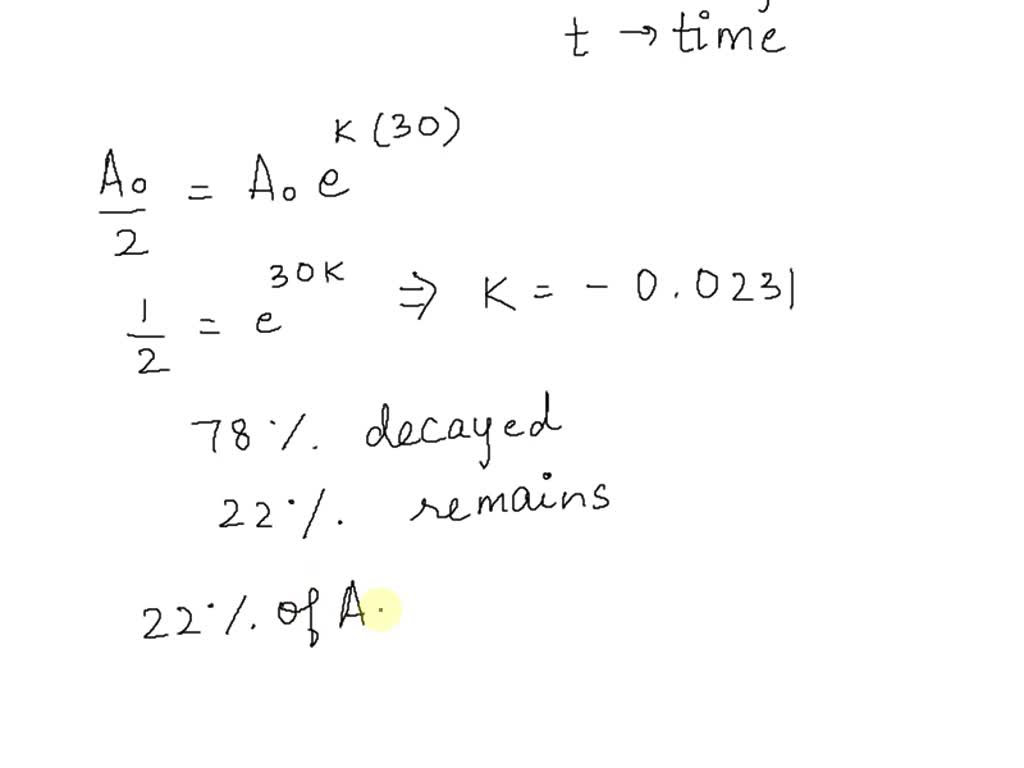
5(t/T) N ( t) = N 0 × 0.However, the half-life can be calculated from the decay constant as follows: half-life = ln (2) / (decay constant). In exponential growth, the rate of growth is proportional to the quantity present.
math quiz Flashcards
The process is random.Explanation: You could use this formula: Where Th = half-life. To find the activity R R using the equation R = 0. Carbon-14 has an abundance of 1.8 g The nuclear half-life of a radioactive isotope tells you how much time must pass in order for half of the atoms present in an initial sample to undergo radioactive decay. If a radioisotope has a half-life of 14 days, half of its atoms will have decayed within 14 days.Given a half-life, explain how you calculate the value of an exponentially decaying quantity at any time t.730 years (t=5. y ′ = − k y 0 e − k t = − k y. That/ Lett be the amount of time that has passed The quantity .

We now turn to exponential decay. is used when there is a quantity with an initial value, x_0 x0, that changes over time, t t, with . The quantity after time is the original quantity times this factor of (9) Ов, Lett be the amount of time that has passed.The rate for radioactive decay is: decay rate = λN. Let t be the amount of time that has passed and Upper T Subscript ha l fThalf be the half-life. Question: Strontium-83 has a half-life of 32. N (0) refers to the initial amount of the . If a quantity decays exponentially, the half-life is the amount . What is the half-life formula calculus? Definition. You will get the calculated time elapsed, i. To measure the decay constant, we take a sample of known mass and measure the number of radioactive decays per second as a function of time. In other words, y′ = ky.5) number of half-lives. Radioactive decay: Scientists . By using the following decay formula, the number of unstable nuclei in a radioactive element left after t can be calculated: N(t) =N0 ×0. Let us start with 200g of the sample After 45 minutes ( first half life) 200 /2 . By comparing the activity of an archeological artifact to that of a sample of the living organism one can estimate the age of the artifact.if a quantity decays exponentially, the half-life is the amount of time it takes the quantity to be reduced by half.25decays (emits a radioactive particle) at . So we have a generally useful formula: y (t) = a × e kt. Thus, for some positive constant k, k, we have y = y0e−kt.25 billion years. 693 N t 1 / 2, we must know N N and t1/2 t 1 / 2.Carbon-14 is decaying constantly with a half-life of 5720 years. Divide ln 2 by the decay constant of the substance.The half-life of this isotope is 10 days.The half-life of a specific radioactive isotope is constant; it is unaffected by conditions and is independent of the initial amount of that isotope.
Half Life Formula
Just as systems exhibiting exponential growth have a constant doubling time, systems exhibiting exponential decay have a constant half-life. Example: 2 months ago you had 3 mice, you now have 18. In this section, we examine exponential growth and decay in the context of some of these applications.00 kg of carbon using the concept of a mole. The half-life of 14C 14 C can be found in Appendix B, and was stated above as 5730 y.0 license and was authored, remixed, and/or curated by OpenStax .
Radioactive Decay Formula
If we can determine the activity of the sample (the number of decays . As long as the organism is alive, the amount of carbon-14 remains relatively constant. with λ = the decay constant for the particular radioisotope. Decay of 210Po, the isotope of polonium in the decay series of 238U, was discovered by the Curies. It is possible to express the decay constant in terms of the half-life, t1 / 2: λ = ln2 t1 / 2 = 0.If a quantity grows exponentially, the time it takes for the quantity to double remains constant. If a spent fuel assembly contains 5.1, and Table 1.The half-life of carbon 14 is 5,730 years.This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. Definition If a quantity decays exponentially, thehalf-lifeis the amount of time it takes the quantity to be reduced by half. To calculate the doubling time, we want to know . This is the energy released per alpha decay. where Nt = N t = activity at time t t and N0 = N 0 = initial activity at .229 × 10 − 3uc2. Choose the correct answer below., 689 years, in the third row, and the sample’s age, i.Whether you are considering radioactive atoms or a population of bacteria, the underlying mathematics is the same: our half-life calculator will teach you how to compute the most important quantity of the decay process, the half-life.
- How Do I Track Someone On Google Maps?
- How Do I Update My Map? – Map Update Service
- How Do You Get A Kanto Starter Pokémon?
- How Do You Use A Comma In A Sentence?
- How Do You Conceptualize A Qualitative Research Question?
- How Do I Log In To The Lake Trust Mobile App?
- How Does Audyssey Save Profiles?
- How Do I Watch The Crossfit Games?
- How Do You Rotate A Vector 90 Degrees?
- How Do You Get The Cult Of Kosmos In Assassin’S Creed Odyssey?
- How Do You Define A Mouth Shape?
- How Do You Convert A Square Meter To Other Units Of Area?
- How Do You Cook Dumplings In A Frying Pan?
- How Do You Remove Chaff From A Grain?