H2O2 Reaction , 21 Facts on HCl + H2O2: With Several Elements Reaction
Di: Samuel
Dissolves readily in dilute acids. Dyke, and Stefano Stranges. Dias, Stefano Falcinelli, Vitali Zhaunerchyk, Edmond P.Hydrogen peroxide, the nonradical 2-electron reduction product of oxygen, is a normal aerobic metabolite occurring at about 10 nm intracellular concentration. Tc for aqueous solns, with pulse heating method to pure H2O2; TRC: Quantity Value Units Method Reference Comment; P c: 220. Use uppercase for the first character in the element and lowercase for the second character.Hydrogen peroxide (H2O2) is a compound involved in some mammalian reactions and processes. In liver, it is produced at 50 nmol/min/g of tissue, which is about 2% of total oxygen uptake at steady state. High selectivity and activity two-electron oxygen reduction reaction (2e− ORR) catalysts are crucial Journal of Materials Chemistry A Recent . The latter process reduces the free-radical yield.Gilbert Torres (UCD) Hydroboration-Oxidation of Alkenes is shared under a CC BY-NC-SA 4. Herein, a triphase photocatalytic system in which the H 2 O 2 generation occurs at the air-liquid-solid joint interfaces is developed, using polymeric carbon nitride . H 2 O 2 is a poor but stable oxidant that fairly reacts with [Fe–S] cluster and loosely bound metals.0 × 10 −6 mol/L/s in a reaction described by the following net ionic equation: 5Br − + BrO − 3 + 6H + 3Br 2 + 3H 2O.
Hydrogen peroxide: a Jekyll and Hyde signalling molecule
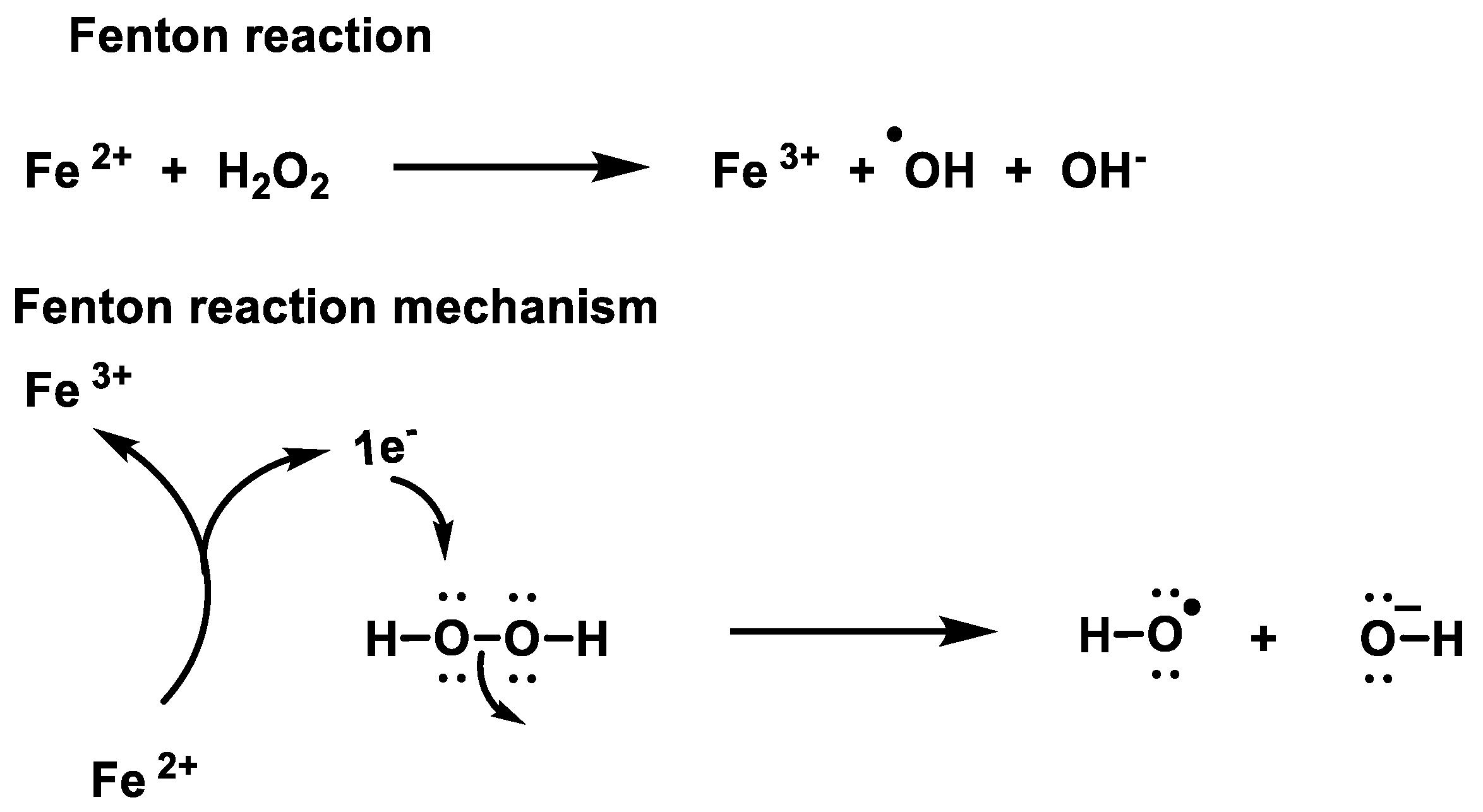
the general rate for this reaction is defined as.The reaction between [Fe(H 2 O) 6] 2+ and H 2 O 2 was thus dubbed the Fenton reaction, while related reactions featuring other metals, ligands and/or peroxides were called Fenton-like reactions .The decomposition of H2O2 to H2O and O2 catalyzed by platinum nanocatalysts controls the energy yield of several energy conversion technologies, such as hydrogen fuel cells. The free radical theory is unable to account for the Fe3+-initiated decomposition of H2O2 or for oxidations by it.In most of its reactions, hydrogen peroxide oxidizes other substances, although it is itself oxidized by a few compounds, such as potassium permanganate.predict the products and specify the reagents for the oxidation of alkynes.0 license and was authored, remixed, and/or curated by LibreTexts.? Comment équilibrer : H2O2 → O2 + H2Operoxyde d’hydrogène, dioxygène, eau Playlist avec de nombreux exemples corrigés pas à pas :https://www.5 h with stirring (1200 r. While not considered a free radical in a strict sense, nonetheless, it is important for its ability to permeate .The rapid charge recombination, low selectivity for two-electron oxygen reduction reaction (ORR), and limited O 2 diffusion rate hinder the practical applications of photocatalytic H 2 O 2 generation. Once the solutions mix, the reaction begins. Cells make the enzyme catalase to remove hydrogen peroxide.First Solution: Starch, Water. The typical reaction conditions used today were developed by G.
Electrochemical Synthesis of H2O2 by Two-Electron Water Oxidation Reaction
Hydrogen peroxide acts as a bleaching agent due to the oxidation of colouring matter by nascent oxygen. Pure hydrogen peroxide freezes at −0.Electrochemical oxygen reduction offers an efficient and environmentally friendly route for hydrogen peroxide (H2O2) generation.
Oxidation-Reduction Reactions
As some curricula do not include this type of problem, the process for balancing alkaline redox reactions is covered on a separate page.0 license and was authored, remixed, and/or curated by Steven Farmer, Dietmar Kennepohl, Layne Morsch, & Layne Morsch.Learn the basics about the decomposition of hydrogen peroxide, as a part of chemical reactions.
The •OH Radical Yield in the H2O2 + O3 (Peroxone) Reaction
Photocatalytic production of hydrogen peroxide (H2O2) on semiconductor catalysts with alcohol as a hydrogen source and molecular oxygen (O2) as an oxygen source has attracted much attention as a potential method for safe H2O2 synthesis, because the reaction can be carried out without the use of explosive H2/O2 mixed gases. Merck’s Method.00: bar: N/A: Nikitin, Pavlov, et al.Consider the reactions: (A) H2O2 + 2HI → I2 + 2H2O (B) HOCl + H2O2 → H3O+ + Cl- + O2 Which of the following statements is correct about H2O2 withThe acid-catalyzed reaction of β,δ-triketones with hydrogen peroxide produces tricyclic peroxides selectively in good yields via the monoperoxidation of the carbonyl groups in β-position and the transformation of the δ-carbonyl group into an acetal.
Hydroboration-Oxidation: The Mechanism
The reaction proceeds in an Anti-Markovnikov manner, where the hydrogen (from \ (BH_3\) or \ (BHR_2\) attaches to . HCl acid is also referred to as muriatic acid. It modulates and signals the redox metabolism of cells by acting as a messenger together with hydrogen sulfide (H2S) and the nitric oxide radical (•NO), activating specific oxidations that determine the metabolic response. The external supply of H2O2 to the Cu(II)/HA system (i. Very hard and brittle.This is called .Oxygen reduction reaction towards hydrogen peroxide (H2O2) provides a green alternative route for H2O2 production, but it lacks efficient catalysts to achieve high selectivity and activity .Electrochemical synthesis of H 2 O 2 represents a growing interest in the distributed production of valuable chemicals with renewable electricity, as evidenced by several recently published reviews on this topic.The reaction was conducted at a temperature of 20°C, for 0. The oxygen produced in 30 seconds is collected over water.H 2 O 2 + 2OH – → 3H 2 O + 2e- +O 2 (Basic medium) 7.
21 Facts on HCl + H2O2: With Several Elements Reaction
This electrochemical process can produce H2O2 on-site under ambient conditions.
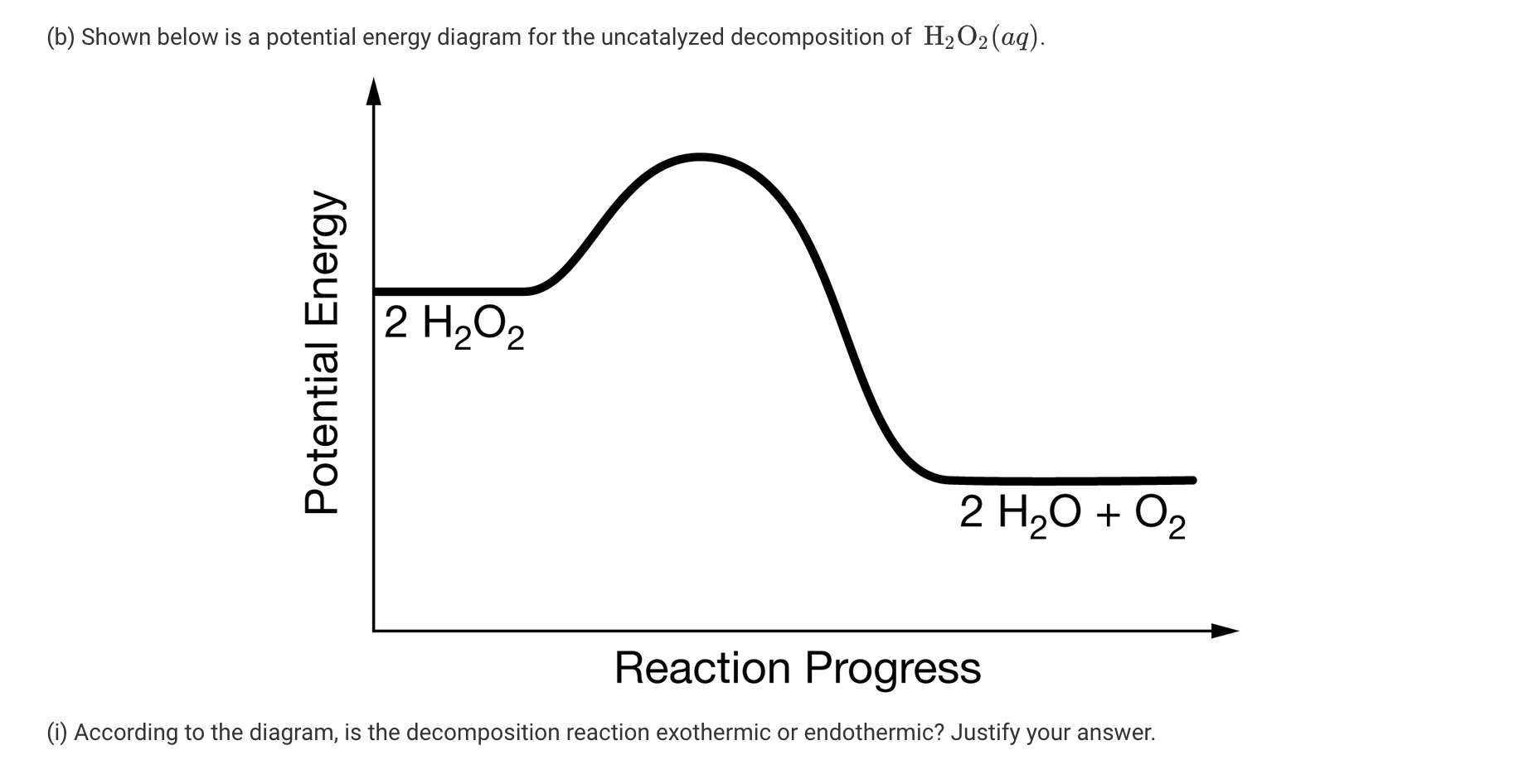
Decomposition of Hydrogen Peroxide
The two-electron water oxidation reaction (2e– WOR) is progressively gaining traction as a sustainable approach for in situ electrosynthesis of hydrogen peroxide (H2O2).1) a A + b B → c C + d D. In appearance, it seems to be colorless, and its molar . The resulting peroxides can be easily isolated from the reaction mixture. For examples, alkynes form vicinal dicarbonyls in neutral permanganate solution. Hydrogen Peroxide Preparation.
Luca Schio, Michele Alagia, Antonio A.Reactive oxygen species (ROS) are a group of molecules produced in the cell through metabolism of oxygen. Here, the authors report CoIn-N-C dual-atom catalyst for effective H2O2 production in acid . Early studies show that direct . In the figure the tangents at t = 15 s have been drawn. 1 The concentration of the dye is plotted time. However, a review focusing exclusively on the electrochemical two-electron water oxidation reaction (2e-WOR) to produce H 2 O .
Characteristic Reactions of Manganese Ions (Mn²⁺)
Pour 1 tablespoon of hydrogen peroxide into a cup. The more downhill the slope of the tangent, the faster the reaction.2010, 44, 3505–3507).A common goal in many of these modifications is the minimization of the H 2 O 2 decomposition reactions (reactions 3 and 4) (). Given a reaction: aA + bB → cC + dD (14. The most common variant of the Iodine Clock Reaction uses sodium thiosulfate (Na 2 S 2 O 3) as the reductant and hydrogen peroxide (H 2 O 2) as the oxidant. By the action of dilute acids on sodium peroxide. Pc for aqueous solns.The mechanistic interpretation for the low • OH yield is as follows (Merényi et al. After the reaction was complete the catalyst was removed from the reaction mixture and two 0. Please draw the products of the following reactions. Write the equations that relate the rates of consumption of the reactants and the rates of formation of the products.La réaction de Fenton est une réaction d’oxydation avancée qui consiste à amorcer des réactions de décomposition du peroxyde d’hydrogène (H 2 O 2) par des sels métalliques afin de générer des espèces radicalaires (HO •, HO •. A Study of H2O2 with Threshold Photoelectron Spectroscopy (TPES) and Electronic Structure Calculations: Redetermination of the First Adiabatic Ionization . Manganese is a gray or reddish-white metal. with pulse heating method to .Balancing reactions under alkaline conditions. Aldehydes and ketones can be converted to . The enzyme is first oxidized to a high-valent iron intermediate, known as Compound I (Cpd I) which, in contrast to other hydroperoxidases, is reduced back to the . Oxygen is present in all parts of the chemical equation and as a result it is both oxidized and reduced . Since alkynes are less stable than alkenens, the reactions conditions can be gentler.This study reports that the combination of Cu(II) with hydroxylamine (HA) (referred to herein as Cu(II)/HA system) in situ generates H2O2 by reducing dissolved oxygen, subsequently producing reactive oxidants through the reaction of Cu(I) with H2O2. Second Solution: Iodine Salt, Reductant, Water. State-of-the-art 2e– WOR electrocatalysts have shown great promise at low electrical currents yet exhibit diminished electrocatalytic capabilities at larger current densities. The rate of formation of Br 2 is 6. The direct reaction of O 2 with fuel is precluded by the .
Oxygen reduction reaction
This article is cited by 11 publications.SUBSCRIBE to the Fuse School YouTube channel for many more edu., the Cu(II)/H2O2/HA system) was found to further .Hydroboration-oxidation converts alkenes into alcohols: THF (tetrahydrofuran) is the solvent that is used to stabilize the BH 3 which otherwise tends to form a dimer, B 2 H 6 – a flammable, toxic, and explosive gas:.A reaction rate can be reported quite differently depending on which product or reagent selected to be monitored. Place the thermometer into the cup. Alkynes, similar to alkenes, can be oxidized gently or strongly depending on the reaction environment. Overall, the carbonyl group is oxidised, whereas the H 2 O 2 is reduced., 1995: Uncertainty assigned by TRC = 20. While the thermometer is still in the cup, dump all the yeast into the cup. Hold the thermometer and the cup so they do not fall over. However, the reaction mechanism and rate-limiting step of this reaction have been unsolved for more than 100 years. Let us discuss HCl and H 2 O 2 reactions. In reactions with Fe2+ ions at high [H2O2], where O2 evolution reaches . Metabolically generated H2O2 emerged from recent research as a central hub in .The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H 2 O 2) in base to form a benzenediol and a carboxylate.0 × 10 −6 mol/L/s in a reaction described by the following net ionic equation: 5Br− +BrO−3 + 6H+ 3Br2 + 3H2O.Reactive Species and Mechanisms of Cell Injury. Rather than combustion, organisms rely on elaborate sequences of electron-transfer reactions, often coupled to proton transfer.
Redox Reaction Calculator
Clementi, in Pathobiology of Human Disease, 2014 Hydrogen Peroxide.
Hydrogen peroxide
Read the temperature and write it down as your “Starting Temperature”. K; by extrapolation of obs.

The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO 2) under mild acidic conditions. HCl and H 2 O 2 are chemical compounds present in an aqueous medium where HCl is a strong acid and hydrogen peroxide is a weak acid.H2O2 is important in large-scale industrial processes and smaller on-site activities.Catalases are ubiquitous enzymes that prevent cell oxidative damage by degrading hydrogen peroxide to water and oxygen (2H2O2 → 2 H2O + O2) with high efficiency. The present industrial route to H2O2 involves hydrogenation of an anthraquinone and O2 oxidation of the .
![[PDF] Decomposition of hydrogen peroxide - kinetics and review of ...](https://d3i71xaburhd42.cloudfront.net/f2948b1ccb4f9f878523a741f22c7a299fa747b2/2-Figure1-1.png)
The 2-electron oxygen reduction in acid is highly attractive to produce H2O2, a vital commodity chemical.Uncertainty assigned by TRC = 15. Such organisms are powered by the heat of combustion of fuel (food) by O 2. Very similar to iron in activity. Endogenous ROS such as hydrogen peroxide (H2O2) have long been recognised as destructive .Enter an equation of a redox chemical reaction and press the Balance button.3 °F) and boils at 150. It was originally developed by Lindgren and Nilsson.0 license and was authored, remixed, and/or curated by James P.00 bar; by extrapolation of obs. Third Solution: Oxidant, Acid, Water. Hydroboration-Oxidation is a two step pathway used to produce alcohols. This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies.
Stresses
Dans la majorité des cas, on aboutit à la formation du radical hydroxyle HO • qui est le . H 2 O 2 → 2 H 2 O + O.Importantly, H 2 O 2 has been identified as the primary product on Au-Pd catalysts at low H 2 conversion (11, 12), whereas the subsequent H 2 O 2 decomposition reactions decrease overall yield as the reaction .
Pinnick later demonstrated . The rate of the reaction at any time equal the slope of the tangent to the corresponding to that time. In the reaction of O 3 with HO 2– an adduct (HO 5–) is formed that decomposes into O 3•– and HO 2• in competition with 2 O 2 + OH –. Colouring matter + O = Colourless matter.Disproportionation reactions have some practical significance in everyday life, including the reaction of hydrogen peroxide, \(\ce{H2O2}\) poured over a cut.2 °C (302 °F); it is denser than water and is soluble in it in all proportions.21 Facts on HCl + H2O2: With Several Elements Reaction.05 g aliquots were titrated against the acidified Ce(SO 4) 2 solution using) with no continual introduction of reactant gasses. It is a few-steps transformation that starts from the addition of borane (BH 3) to the alkene. We determined both the reaction mechanism and .
Pinnick oxidation
The balanced equation will be calculated along with the oxidation states of each element and the oxidizing and reduction agents.The oxygen reduction reaction is an essential reaction for aerobic organisms.
Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Measure 1 teaspoon of yeast.
9: Nucleophilic Addition of Hydrazine – The Wolff-Kishner Reaction is shared under a CC BY-SA 4.This page titled Characteristic Reactions of Manganese Ions (Mn²⁺) is shared under a CC BY-NC-SA 4. Working out half-equations for reactions in alkaline solution is decidedly more tricky than the examples above.Hydrogen peroxide (H2O2) is usually considered to be an important reagent in green chemistry since water is the only by-product in H2O2 involved oxidation reactions. This a decomposition reaction of hydrogen peroxide, which produces oxygen and water.Consideration of the changes in free energy shows that the assumed initial steps in reactions of H2O2 with Fe2+ and Fe3+ in the free radical theory are not consistent. Examples: Fe, Au, Co, Br, C, O, N, F. This article was most recently .
- Gw2 Behälter Mit Eingefangenem Blitz
- H U M Online | Von uns akzeptierte Zahlungsmethoden
- Guten Wie Schweren Tagen Film _ In guten wie in schweren Tagen: Ähnliche Filme
- Gute Vertriebspraxis Leitlinie
- Haare Aus Spenderbereich – Haartransplantation Spenderbereich
- Gymnasium St Xaver Paderborn _ „Music is my first love!“
- Haftpflichtversicherung Hagelschaden
- H7 White Led : H7 LED E39
- Hafencity Führungen : Elbphilharmonie Führung
- Hackfleisch Fettgehalt Tabelle
- Haferflocken Für Hunde Geeignet