Echa Biocides Database | What’s in a biocide?
Di: Samuel
The database is refreshed regularly with new and updated notifications. Product type 2 includes disinfectants not intended for direct application to humans or animals, while product type .According to a news article published by ECHA on October 5, 2015, PHMB (1415; 4. AM0573/00 Hydroplus Wood protection insectiside.Zulassung von Biozidprodukten.Biocides are products that act against pests and bacteria. Directorate of Energy, Industrial and Chemical Products – DG of General Chemical State Laboratory under the Independent Authority for Public Revenue (IAPR) 16, An. It also includes the list of harmonised classifications. Hier finden Sie Informationen über Biozidprodukte, die gemäß der Richtlinie über Biozidprodukte (Richtlinie 98/8/EG) oder der Verordnung über Biozidprodukte (Verordnung (EU) Nr. Biocides are also important in many industrial processes to prevent microorganisms from growing. It also explains the guiding principles for the evaluation of the applications to be performed by the authorities. This is ECHA’s new public chemicals database with information from all REACH registrations received by the Agency.
R4BP 3
The current guidance focuses only on bees due to the lack of data on non-bee . When a person uses the ECHA website, the person (hereinafter the “User”) fully agrees . Join our IT days second edition in Helsinki. bodies, and agencies. In 2022, ECHA announced that it would create a new system for publishing chemicals data.You can see the status of biocides assessments on the Biocidal Active Substances page. Therefore, companies are required to inform consumers about these substances in their own language on the labels of the products. You must indicate the expiry date and the production date on the label, and if necessary give such expiry dates for different climate zones.In 2019, the European Commission requested ECHA to develop a guidance for assessing how biocides exposure affects arthropod pollinators, including bees. ECHA makes available this information as REACH registered substance factsheets, which contain the full set of non-confidential . It covers information on active substances for which an application for approval for a specific biocidal product-type has been submitted under the BPR or Biocidal Products Directive (Directive 98/8/EC). Elle comprend également la liste des classifications harmonisées. In the database, you can see the status of each substance in combination with product type.For this reason,ECHA organises an online training session tailored to industry applicants. The subsequent steps in processing such applications will not be covered .
Nationale Zulassung und gegenseitige Anerkennung. Start date: 01/03/2024. The aim of the Biocides Submission Manual (BSM) series is to provide industry users with detailed and illustrative technical assistance. Please be aware that ECHA does not verify . The event is targeted at IT professionals and gives the opportunity to learn what is happening in our IT landscape and transformation, get inspired by keynote speakers, share on trending themes in the IT world, and connect with your . This has been done as part of actions under the EU biodiversity strategy for 2030 and the Pollinators Initiative . Apologies for the inconvenience. The first version of this TNsG is from 2000.The use of biocides in animal husbandry can lead to the exposure of livestock.
Can we trust the labels on products treated with biocides?
ECHA’s current Information on chemicals platform, launched in 2016, grew rapidly and contains today information on over 360 000 chemicals. The BSM series describes how to build IUCLID dossiers for the various Biocidal Product Regulation applications and how to submit and manage those applications in R4BP 3 .eu Contact Points: Catalogue Record: Added to data. AM0573/00 Hydroplus protettivo per legno insetticida. Applicability of GuidanceGuidance on BPR: Volume III Parts B+C Version 4.0 December 2017 2 LEGAL NOTICE This document aims to assist users in complying with their obligations under the Biocidal Start date: 07/02/2024.Biocides Submission Manuals. GR-11521 Athens.Guidance on PIC. A guideline has been prepared on European level to estimate the exposure of agricultural livestock to biocidal active substances (ECHA Guidance on the Biocidal Products Regulation, Volume III, Parts .Fax: +30 210 64 66 917. The Biocidal Products Regulation (BPR, Regulation (EU) 528/2012) concerns the placing on the market and use of biocidal products, which are used to protect humans, animals, materials or articles against harmful organisms like pests or bacteria, by the action of the active substances contained in the biocidal product.Biocidal products that have been granted a so-called Union authorisation are listed in the ECHA database of biocidal products.Identification of substances of very high concern. The Biocides team.This Introduction includes general information that is applicable to the Part A of volumes I-IV and contains general guiding principles relevant for all four Volumes. Disclaimer The information and views set out in the ED assessment list and in the hazard assessment outcome documents are those of the evaluating authority and do not necessarily reflect the position or opinion of the other Member States or ECHA.The data on active substances is collected from the Register for Biocidal Products (R4BP 3).In this table, you will find all public data submitted to ECHA in REACH registration dossiers by substance manufacturers, importers, or their representatives, as laid out by the REACH Regulation (see Understanding REACH regulation).Biocides are crucial for preventing and controlling the spread of infectious diseases in hospitals and other health facilities. This means that you can see whether the active substance is approved or is still undergoing evaluation for a certain product type. Guiding principles on information requirements in general. EU law European data EU tenders .Vous pouvez exporter les résultats relatifs aux produits biocides vers les formats standard du site web de l’ECHA (XLS, CSV et XML). Please note that the validation step in R4BP 3 must be . Les résultats exportés sont classés par autorisation de produit, c’est-à-dire que chaque autorisation est présentée dans une ligne individuelle.
Biocides supplier or user
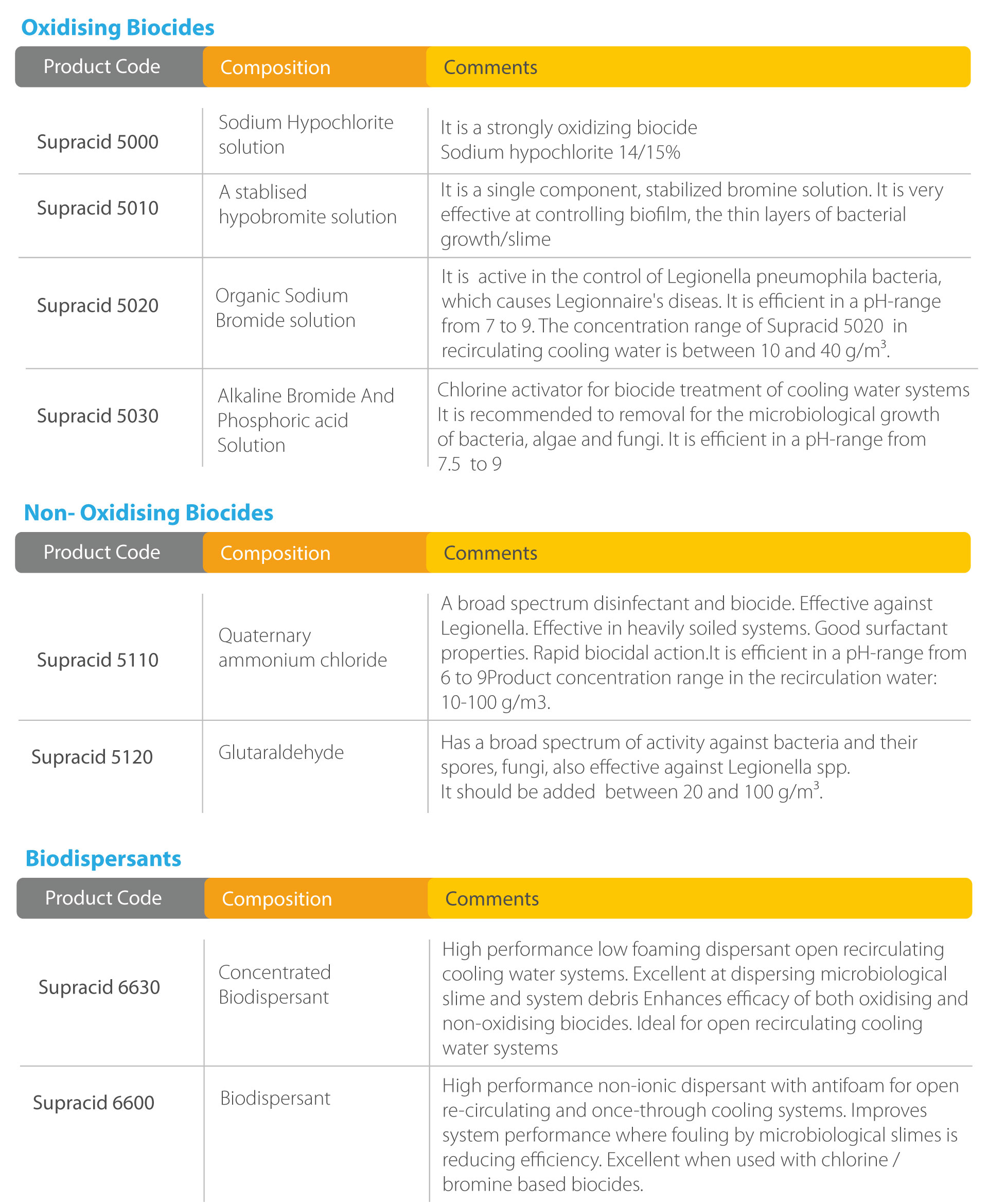
Aqua Madeiras Protect Plus.This Guidance replaces the addendum “Technical Notes for Guidance on data requirements for micro-organisms including viruses and fungi (EU, 2005)”, to the Technical Notes for Guidance (TNsG) on Data Requirements (EU, 2008a) in support of Directive 98/8/EC (Biocidal Product Directive – BPD).458-0005/2 IDRO CEOPREN Wood protection Insecticide. Importing, manufacturing or selling? If you want to import or sell a biocidal product in Sweden, the product must be authorised by a competent authority in the EU.ECHA organises consultations to get feedback from all interested parties and to gather the widest possible range of scientific information for the regulatory processes.R4BP 3 is the central hub through which all biocides’ applications are submitted to ECHA and National Authorities. This database contains classification and labelling information on notified and registered substances received from manufacturers and importers.European Commission decisions on approval and non-approval are published in the Official Journal of the European Union.Search for articles (products) in SCIP database. In questa sezione figurano informazioni sui biocidi autorizzati sul mercato dell’UE/SEE in conformità della direttiva sui biocidi (direttiva 98/8/CE, BPD) o del regolamento sui biocidi [regolamento (UE) n. Other Services. Tale autorizzazione avviene in due fasi consecutive. As a result of excluding biocidal products used as preservatives for food and feedstock from the scope, there is one less product type compared to the previous directive.
Zulassung von Biozidprodukten
Cette opération se déroule en deux étapes .On the ECHA website for biocides, there is a database of active biocidal substances.Furthermore, you must package and label the exported chemicals – including all exported biocides irrespective of whether they are banned or severely restricted within the EU – according to the CLP Regulation. The following guiding principles reflect the general guidance on information requirements which.
ECHA CHEM
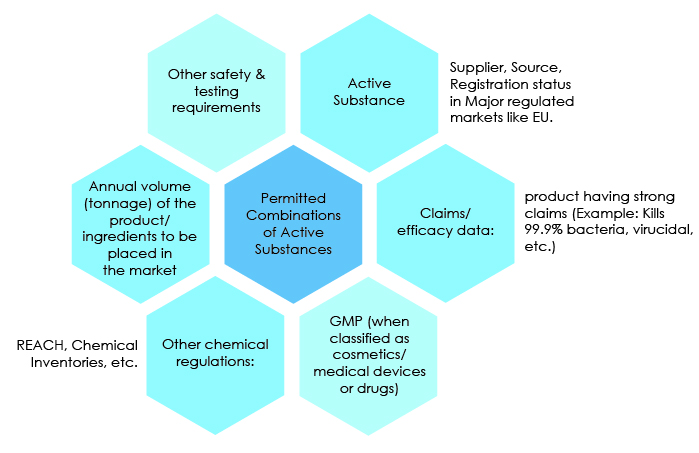
Exposure estimation for biocides
The European Chemicals Agency (hereinafter “ECHA”) maintains this website (hereinafter the ECHA website) to enhance public access to information about its activities, and to fulfil its obligations falling under its mandate. To have this effect, they often contain hazardous chemicals.

ECHA CHEM will be gradually enriched with more information on chemicals.ECHA R4BP pages; National Helpdesks R4BP news .
Biocidal Products Directive
The TNsG on Human Exposure document (2007 *) provides guidance on the estimation of Human Exposure to biocidal products for all Product Types. Alle Biozidprodukte, die genehmigte Wirkstoffe enthalten, werden auf Sicherheit und .
Le principe de base du règlement sur les produits biocides [règlement (UE) nº 528/2012 (RPB)] est qu’un produit biocide doit être autorisé avant de pouvoir être mis à disposition sur le marché ou utilisé dans l’Espace économique européen (EEE) et en Suisse.
![]()
AQUA STAIN PRIME 2030-25.
Endocrine disruptor assessment list
7) may be approved for product types 2 and 4, but not for 1 (human hygiene), 5 (drinking water), and 6 (preservatives for products during storage). Tutti i biocidi contenenti principi attivi approvati sono valutati sotto il profilo della . Il principio di base del regolamento sui biocidi [regolamento (UE) n. Box 400, FI-00121 Helsinki, Finland . Substances of very high concern identification; Draft recommendation for inclusion in the Authorisation List and consultation; Applications for authorisationBiocides may give your every day products desirable properties but some of the substances in them can be hazardous. An enforcement project with national enforcement authorities from 22 different EU Member . Aqualasur Guard. The consolidated version of the Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products (BPR) incorporates all of the amendments and corrigenda to the BPR adopted to date. Helsinki/Online | 29/05/2024 – 30/05/2024 | 09:00 – 17:00.Base de données de l’inventaire C&L.
Understanding BPR
The most recent version is of 2008 (which includes an Excel file containing default exposure data for all PTs). Search the database on the ECHA website External link. They help restaurants and the food industry keep harmful pathogens out of our food and ensure our drinking water is safe. Unternehmen können zwischen verschiedenen alternativen Verfahren wählen, je nach Produkt und Anzahl der Länder, in denen das Produkt vertrieben werden soll. Fax: +30 210 64 66 917.
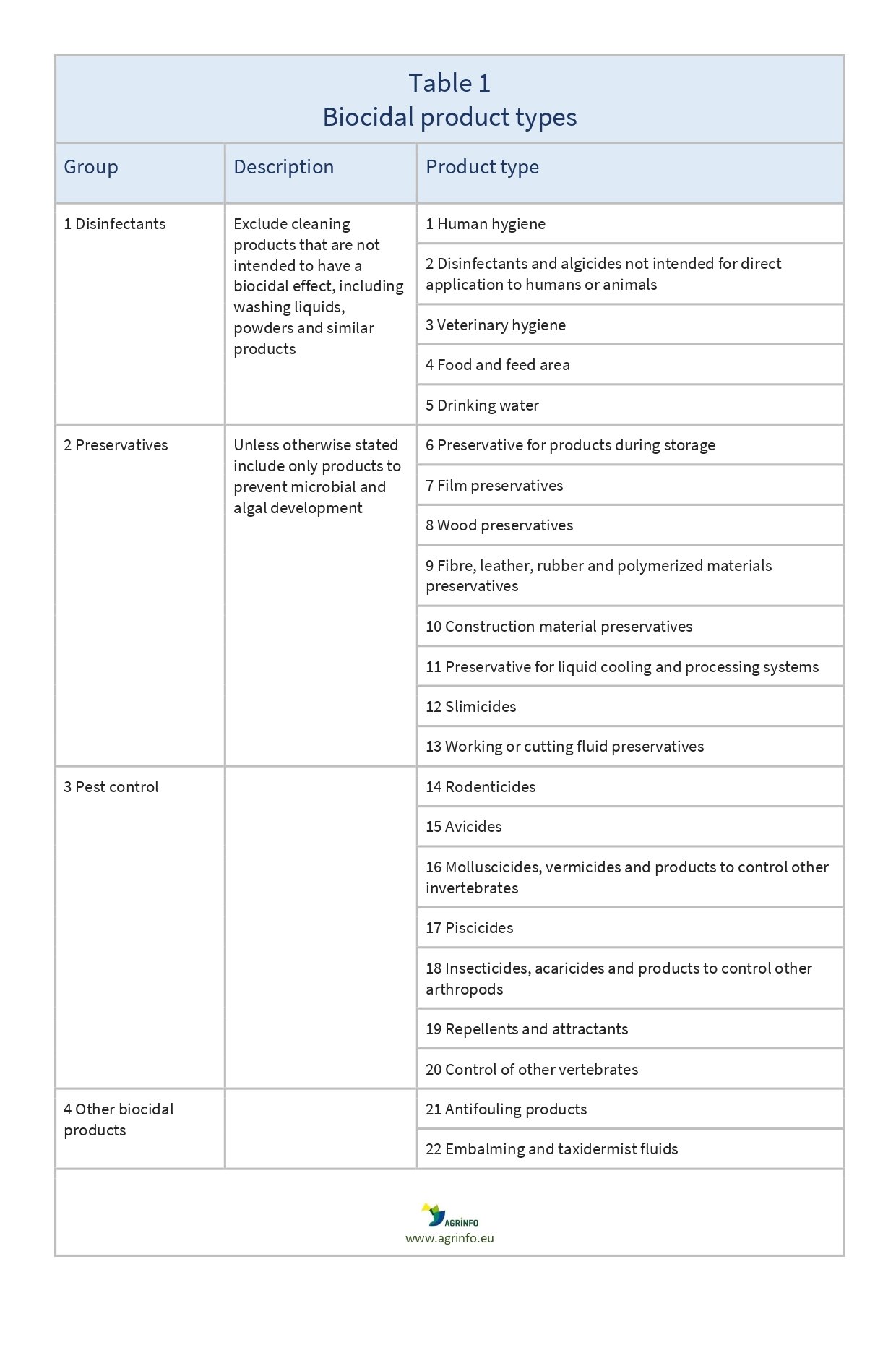
Product-types
528/2012) auf dem EU-/EWR-Markt zugelassen sind. Deadline: 15/04/2024. The ECHA Guidance on biocides legislation describes how to fulfil the information requirements set by the Biocidal Products Regulation, Regulation (EU) 528/2012) (BPR) and how to perform the required assessments.Biocidal products may be made available on the German market and used in the following cases: on the basis of transitional measures during the approval procedure of the contained active substance(s) (Article 89(2) of Regulation (EU) No 528/2012 (Biocidal Products Regulation) in conjunction with section 28(8) of the German Chemicals Act and the . A new version of the legal notice is available. As such, it is a central point of access to EU law, publications, open data, research results, procurement notices and other official information. The use of such information may therefore require the prior permission of the third party owners.
Volume V, Guidance on Active Micro-organisms and Biocidal Products
Information on biocides.

Recommendation for inclusion in the authorisation list.ECHA – Biocidal Products Regulation The Biocidal Product Regulation (BPR, Regulation (EU) 528/2012) concerns the placing on the market and use of biocidal products, which are used to protect humans, animals, materials or articles against harmful organisms, like pests or bacteria, by the action of the active substances contained in the biocidal product.
What’s in a biocide?
The scope of the session will include data preparation in SPC-IUCLID and some simple steps in R4BP 3 to perform the submissions of applications for product authorisation. 674810 HF ACTIVE PRIMER W. 528/2012 (BPR)] è che un biocida deve essere autorizzato prima di poter essere immesso sul mercato o utilizzato nello Spazio economico europeo (SEE) e in Svizzera. Telephone: +30 210 64 79 286; +30 210 64 79 287.eu: 03 December 2021. European Chemicals Agency Telakkakatu 6, P. Please note that some of the information on chemicals may belong to third parties. Please consult the Legal Notice for further information.
The database also shows decisions .Homepage: https://echa.
Biocidal products
In Annex V to the BPR the biocidal products are classified into 22 biocidal product-types, grouped in four main areas. You must read and accept it to .
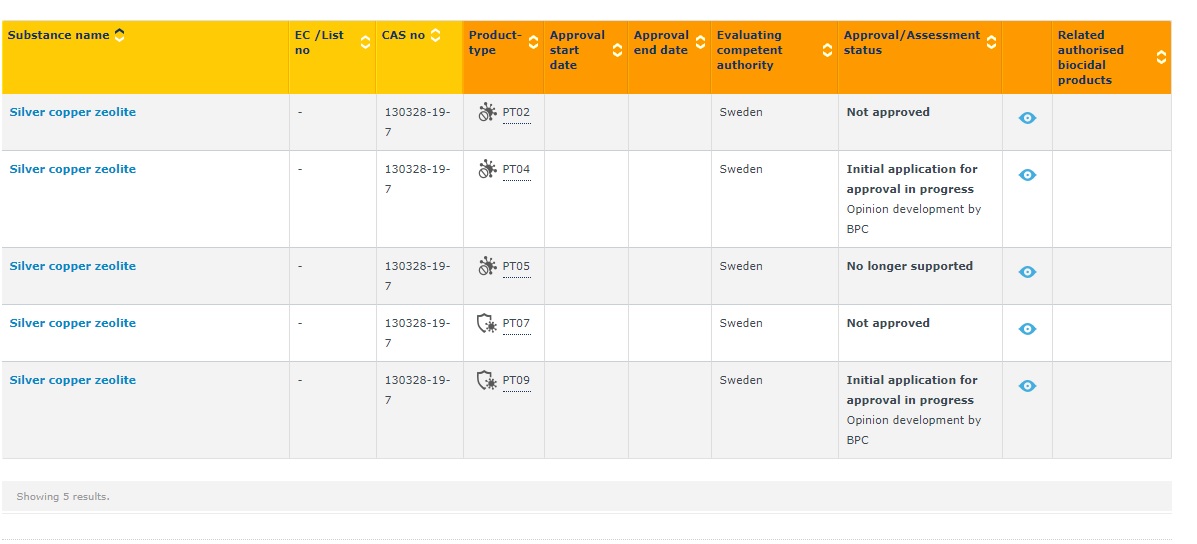
It provides functions which enable the industry and the authorities to comply with the legislative requirements and exchange information between them. As biocidal active substances are approved, there is an obligation on ECHA to make certain non-confidential data submitted as part of the process of obtaining the approval available. Biocides submissions under the Biocidal Products Regulation (BPR, Regulation .
Information on biocides
Alle Biozidprodukte müssen zugelassen werden, bevor sie in Verkehr gebracht werden. Deadline: 07/05/2024. Email: elhelpdesk (at) gcsl. Recommendation to amend authorisation list . If you want to know what’s in the biocides you buy and to find the most environmentally friendly options, try out our database on biocides. Welcome to ECHA CHEM. These product types exclude cleaning .

Biocides Submission Manuals
Understanding BPR. ECHA CHEM allows the Agency to better handle the growing diversity and quantity of data, while taking advantage of . Search SCIP database. This may lead to the formation of residues in foods of animal origin.
C&L Inventory
Read more about the transition on ECHA website. 528/2012, BPR]. AA1961 Laqvin Seal Insecticide. Cette base de données contient des informations relatives à la classification et à l’étiquetage concernant les substances enregistrées et ayant fait l’objet d’une notification, reçues des fabricants et des importateurs.Consolidated version of the Biocidal Products Regulation. However, updated notifications cannot be specifically . La colonne «target organism» (organisme cible) du . This 2000 version was revised two times.
- Easysupport Für Fritzbox – EasySupport
- Echte Dating Seiten : Die 4 beliebtesten komplett kostenlosen Singlebörsen
- Edeka Hahner Höchstadt Prospekt
- Edeka Bio Tomatenmark : Pesto Rosso
- Easy Themen Zur Präsentation _ Mahatma Gandhi Steckbrief
- Edeka Burkowski Bochum Spargel
- Ebay Zu Verschenken Plattling : Spiegelschrank Regal Kommode Schrank zu verschenken
- Edeka Vogel Lewandowski Angebote
- Ecological Art Wikipedia _ Ecocriticism
- Ed Sheeran Beste Songs _ Die 10 schönsten Songs von Ed Sheeran
- Ecm Siebträger Entkalken – Entkalkung einer ECM Classika