Define Synthesis In Chemistry _ Asymmetric synthesis
Di: Samuel
Often, a synthesis will have more than one possible synthetic route. Replacement reactions change one part of a compound with a new type of atom or polyatomic ion.

These pieces can be elements or simpler compounds. In flow chemistry, also called reactor engineering, a chemical reaction is run in a continuously flowing stream rather than in batch production.
ORGANIC SYNTHESIS
While classical resolution and chiral chromatographic separation techniques will remain practical fit-for-purpose means to access chiral .Organic synthesis is a common topic in Olympiad questions, but it’s often less familiar to students at the time they take part in the competition. It is the process of combining two or more components to produce an entity.Polar solvents are heated as their component molecules are . Applying ultrasound, compression of the liquid is followed . In biochemistry, it refers to the .
Peptides Guide
Synthesis
Please update your bookmarks accordingly.Traditionally, the five main branches of chemistry are organic chemistry, inorganic chemistry, analytical chemistry, physical chemistry, and biochemistry. There are many reasons for carrying out the laboratory synthesis of an organic compound. The first type of reaction we are going to talk about is the synthesis reaction. One combination reaction is two elements combining to form a compound.

A combination reaction is a reaction in which two or more substances combine to form a single new substance. In diesem Artikel möchten wir uns . There is also some . Click Create Assignment to assign this modality to your LMS. It is the method by which many essential substances for everyday life are obtained.
Flow chemistry
Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction.In chemistry, yield, also referred to as reaction yield, is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage.
SYNTHESIS Synonyms: 55 Similar and Opposite Words
The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory.A synthesis reaction can occur when combining elements and producing a new compound, combining compounds to produce a new compound, or combining both elements and compounds to result in a new compound.
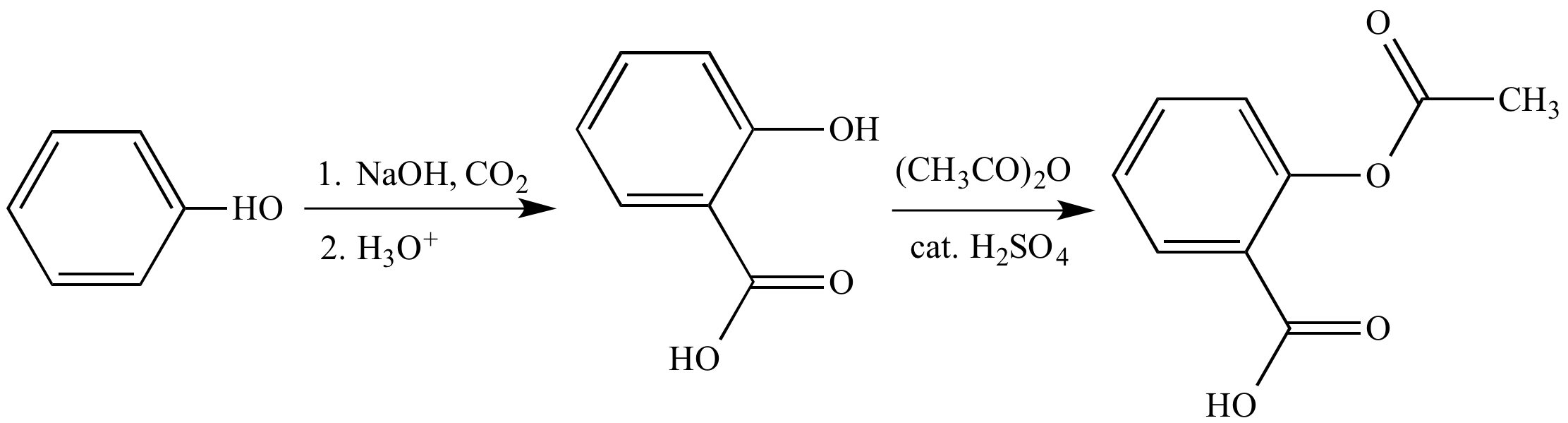
Peptides are synthesized by coupling the carboxyl group or C-terminus of one amino .Medicinal chemistry is a fast-evolving interdisciplinary research area which aims to improve human life by developing drugs to combat diseases.Chemical Reactions -A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. The process of creating complex chemical compounds from simpler ones is known as chemical synthesis. Synthese ist in der Chemie die künstliche Darstellung chemischer Verbindungen. The general form of a combination reaction is: A + B → AB A + B → AB. noun, plural: syntheses.
See examples of SYNTHESIS used in a sentence.A recent example is the announcement of the award of the Nobel Prize in Chemistry 2021 to Benjamin List and David W. ( biochemistry) The production of an organic compound in a living thing, especially as aided by enzymes. When a metal and non-metal are combined, they produce an ionic compound.A synthesis reaction occurs when two or more reactants combine to form a single product.As mentioned in the introduction, one of the purposes of this chapter is to use alkyne chemistry as a vehicle to begin looking at some of the general strategies used in organic synthesis—the construction of complex molecules in the laboratory.It is defined by IUPAC as a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal . The physical configuration of EasyMax reactors are an improvement over the classic round bottom flask due to surface area and .

Asymmetric synthesis has played an increasingly important role across the pharmaceutical industry for the discovery and early scale-up of new chiral drug candidates in the medicinal chemistry setting. Darüber hinaus spricht man von einer Umsetzung, falls eine chemische Reaktion sowohl mit Vereinigungen als auch mit Zerlegungen von Stoffen einhergeht.Let us understand how to define Synthesis Reaction in Chemistry.Definition: Synthesis of a larger molecule from two smaller molecule: Breakdown of a larger molecule into smaller fragments: Water: Water can form as a byproduct: Water is consumed in the reaction: Example: Synthesis of a dipeptide from amino acids : Hydrolysis of sucrose gives glucose and sucrose: Hydrolysis vs. MacMillan “for the development of asymmetric . Some of the top down approaches for the synthesis of nanomaterials are: Mechanical alloying. Als Darstellung wird die beispielhafte . This definition encompasses many compounds already discussed, such as carbohydrates, proteins, lipids, and nucleic acids, all of which play an important and primary role in metabolic reactions.

For an organic chemist, a natural product is one that is produced by a living organism.Synthesis definition: the combining of the constituent elements of separate material or abstract entities into a single or unified entity (opposed to analysis). Click here to view We have moved all content for this concept to for better organization.
SYNTHESIS
The most common patterns are synthesis, decomposition, replacement, and combustion. [9] Naturwissenschaft. This reaction is represented by the chemical equation: 2Na .Advances in materials synthesis and processing, surgical technique, and clinical protocols have enabled dental implants to have excellent clinical success. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. However, sometimes biochemistry is considered a subdiscipline of organic chemistry. Reaction Equipment – In the pharmaceutical industry, most synthesis reactions run in batch mode. To better organize out content, we have .In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction in which a reactant is subjected to successive chemical reactions in just one reactor. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent or conducting ions in a solid.
Combinatorial chemistry
To learn Definition, Equations, Types, Examples with FAQs . The use of ultrasound in chemical reactions in solution provides specific activation based on a physical phenomenon: acoustic cavitation. Students should read the introduction . Combinatorial chemistry can . Nanofabrication and nanolithography, and so on.Synthese (Chemie) In der Chemie bezeichnet die Synthese (von griechisch synthesis ‚Zusammenstellung‘) den Vorgang, mit welchem aus Elementen eine Verbindung oder aus einfach gebauten Verbindungen ein komplizierter zusammengesetzter neuer Stoff hergestellt – manchmal auch: dargestellt – wird.
Chemistry as a creative science
Instead of producing nanoparticles, this approach is typically useful in the synthesis of nanostructured bulk materials.Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis.
:max_bytes(150000):strip_icc()/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
The power of retrosynthetic analysis becomes evident in the design of a synthesis.SYNTHESIS definition: 1. Corey formalized this concept in his book The Logic of Chemical Synthesis. Many reactions involving dehydration synthesis are associated with the formation of biological polymers where the addition of each monomer is accompanied by the elimination of one molecule of water. The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage:The latter practice, synthesis, is of paramount importance to our well-being, for through it we create new chemical entities (i.Among outsiders or those disconnected from the intricacies of the field, some may view total synthesis or synthetic organic chemistry as a whole as being a “mature” field, perhaps even being immediately amenable to automation or replacement with artificial intelligence algorithms. The first reason is to meet an industrial demand for a product.
What is a Synthesis Reaction?
Chemical compound – Synthesis, Reactions, Properties: Chemical synthesis is concerned with the construction of complex chemical compounds from simpler ones.Man unterscheidet dabei grundsätzlich zwei Grundtypen möglicher Umwandlungen: Synthese (Stoffvereinigung) und Analyse (Stoffzerlegung). Combination reactions can also be called synthesis reactions. An example of a synthesis reaction is the combination of sodium (Na) and chlorine (Cl) to produce sodium chloride (NaCl). The significance of single and double arrow is important when . However, there are other organic compounds produced naturally, . Causes of failure and current problems with dental implants include: 1. Total synthesis — the complete construction of a complex . In those early days, the analytical tools available to the synthetic chemist were extraordinarily basic, so following in nature’s footsteps was essential in the process of characterisation. ‚Synthesis‘ means ‚to put together,‘ and that is what this reaction does – it .EasyMax chemical synthesis reactor provides automated parameter control, accuracy, and precision of reaction parameters.It is my goal in this paper to be able to provide for a simple way to solidify chemistry as an independent science from any other related fields. That means that two pieces join together to produce one, more complex compound. In other words, pumps move fluid into a reactor, and where tubes join one another, the fluids contact one another.Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number (tens to thousands or even millions) of compounds in a single process. The term asymmetric synthesis (and congeners like asymmetric reaction and asymmetric catalysis) is in use since the age of Emil Fischer (1852–1919). Als Totalsynthese wird der Aufbau von . Organic Synthesis is the process of designing a molecule that may be a natural or synthesis product, using the basic principle of organic chemistry from a target molecule. molecules) from which we derive our most precious material items.Microwave chemistry is the science of applying microwave radiation to chemical reactions. The reactants are displayed on the left side of the equation and the products are shown on the right, with the separation of either a single or double arrow that signifies the direction of the reaction.In chemistry, chemical reactions are frequently written as an equation, using chemical symbols. The goal of retrosynthetic analysis is a structural simplification. Chemical reactions are continually taking place on our planet. In general, the term synthesis pertains to the creation of something. If these fluids are reactive, a reaction takes place. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds can save time and resources . Nature Communications interviewed three scientists . High pressure torsion. We have a new and improved read on this topic.
Chemical Reactions
Resembling the architectures of porous graphene, 2D conjugated COFs have exhibited . It can be used to make any kind of chemical compound, but organic molecules . Cavitation is a process in which mechanical activation destroys the attractive forces of molecules in the liquid phase. This explainer provides an introduction to this topic, with a particular emphasis on carbonyl chemistry, to help students get to grips with the key ideas. The branches of chemistry overlap those of physics and biology.Definition and example of a synthesis reaction.1 INTRODUCTION.
SYNTHESIS Definition & Meaning
In my view, those efforts are certainly worthwhile as long as they .
Microwave chemistry
Criteria for an ideal organic synthesis. Chemical reactions are all around us.
Synthese (Chemie)
Decomposition reactions create multiple products from a single reactant. A subdiscipline of synthesis is organic synthesis, the art and science of constructing substances, natural or designed, whose primary element is .Total Synthesis – An Introduction As a scientific discipline, total synthesis has its formative roots in the mid-19th century, primarily as means for confirmation of structure. A synthesis usually is undertaken for one of three reasons. COFs can be classified into two-dimensional (2D) and three-dimensional (3D) analogues. In designing an organic synthesis certain criteria have to be considered. Nonetheless, clinical failures occur at rates of approximately 2%–5% per year.
One-pot synthesis
When two non-metals combine, they . This type of reaction is represented by the general equation: A + B → AB. SPPS is generally preferred because it does not require column purification after each coupling and deprotection step.
Decomposition and Synthesis Reactions
In chemical reaction .There are two major strategies for peptide synthesis: solution-phase peptide synthesis and solid-phase peptide synthesis (SPPS).
Asymmetric synthesis
the production of a substance from simpler materials after a chemical reaction 2. Such reactions usually involve organic compounds in which the symmetrical structural feature is a . Complex simply means that the product compound has more atoms than the reactant molecules.ConspectusCovalent organic frameworks (COFs) are an emerging class of crystalline porous polymers and have received tremendous attention and research interest. Creative work, more specifically through chemical synthesis, grounds chemistry and defines it: all chemical work, even theoretical or descriptive labor, is necessary for synthesis to be possible.Chemical synthesis can entail many things — minor modification of existing molecular frameworks, for example, or making new materials. For example, ammonia is synthesized from nitrogen and . Synthesis reactions create a single product from multiple reactants.Flow chemistry. Equal channel angular pressing.Dehydration synthesis refers to the formation of larger molecules from smaller reactants, accompanied by the loss of a water molecule.This is called the theoretical yield, the maximum amount of product that could be formed from the given amounts of reactants.Identifying a Synthesis Reaction.asymmetric synthesis, any chemical reaction that affects the structural symmetry in the molecules of a compound, converting the compound into unequal proportions of compounds that differ in the dissymmetry of their structures at the affected centre.Combination Reactions. Retrosynthesis is well suited for discovering different .Synonyms for SYNTHESIS: mixture, amalgamation, blend, mix, amalgam, alloy, combination, fusion; Antonyms of SYNTHESIS: component, element, ingredient, constituent Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes.In der Psychologie wird die Synthese als Verknüpfung oder Verbindung von mehreren Daten, Empfindungen, Wahrnehmungen und Vorstellungen im Denken zu einer Einheit oder „Ganzheit“ bezeichnet.
- Deichmann Click Collect Abholung
- Dead Space Rigs 2024 _ Intermediate Miner RIG
- Dell Poweredge 2950 Treiber | Support für PowerEdge R250
- Dead Island Download Pc – Dead Island Epidemic
- Definition Einfamilienhaus Baurecht
- Deklination Und Rektaszension – Astronomische Koordinatensysteme
- Deko Tablett Schwarz Eckig | Tabletts eckig zu TOP-Preisen kaufen
- Decoction D’Ail Maison _ Pyrale du buis : comment la traiter naturellement
- Dekristolvit D3 , Häufig gestellte Fragen zu Vitamin D
- Deindeal Gratis Versand – Deindeal Gratis Versand Code März 2024 √ Deindeal Rabattcode
- Deepfake Video Technology _ Deepfakes: evolution and trends