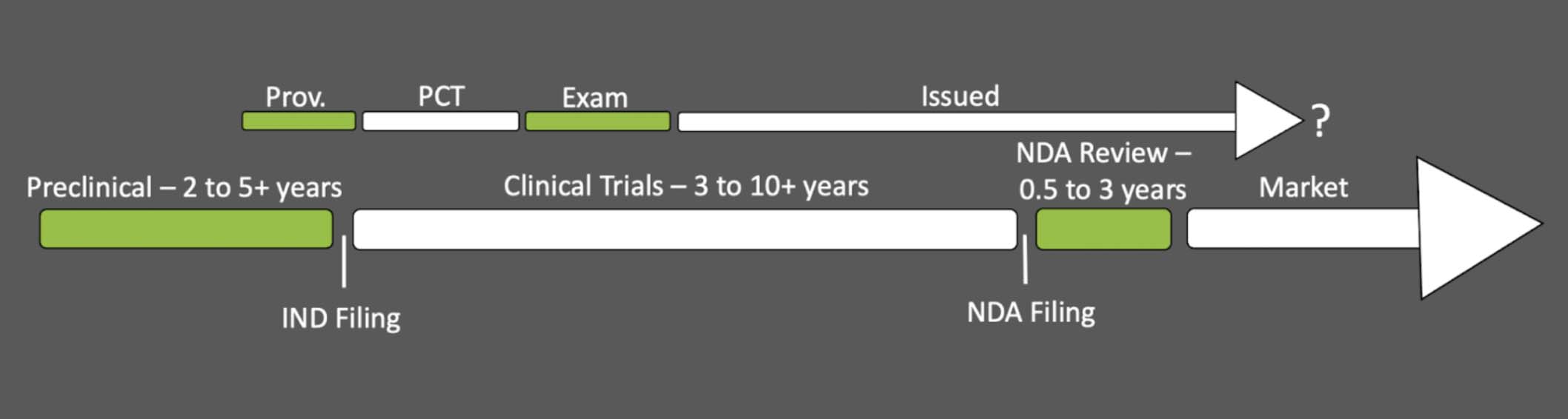
Data Exclusivity 20 Years : (PDF) Protection of Data in China: Seventeen Years after
Di: Samuel
2 Thus, Article 20. the terms of patents covering existing products. Footnote 73 In addition, the agreements with a number of European countries require a . Footnote 64 The Indian law does not connect the definition of a “new drug” with its patent status but defines it as a drug which has not been used in the . It applies in relation to therapeutic goods which contain a “new active component” which has not been previously . “the 8+2 regime”. Data exclusivity is a form of .
DATA PROTECTION FOR BIOLOGICS

But in data exclusivity, the right holder has no like monopoly since the generic companies have opportunity getting marketing approval by presenting their test results and clinical trials 15 even though the . Indian regulations, like those in the United States . Invalidation therefore constitutes a natural experiment that allows us to identify how the duration of market exclusivity affects firms‘ incentives to innovate. Such market exclusivity shall start from the date when the first orphan MA, for a same active substance, was granted in the Union. when it’s applied . The new rules would also allow competitors to file 2 years before the expiry of orphan exclusivity, which they cannot currently do.6 +1 year market protection for a new therapeutic indication which brings significant benefit in comparison with existing therapies (Art. In order to apply for an extension it must be shown that there was “unreasonable curtailment” of the opportunity to exploit the patent .21 USC 355 (c) (3) (E) (ii,iii), which set out the period of data exclusivity under US law. They are an important and innovative development in the pharmaceutical industry and a step towards the .Today, section 520 (h) (4) of the FDCA provides six years of data exclusivity for medical devices approved pursuant to a PMA. By Rachel Thrasher.Data Exclusivity. 9 years for others orphan medicinal products. That is normally true, except in one important circumstance. At market approval, a new drug enjoys concurrent protection from the remaining patent term and from the fixed period of data exclusivity.to market” the follow-on product for the specified time period.

A company would also receive three years of market protection from generics, taking the total exclusivity period to a maximum 11. From 2017, a new .This further two-year period means that there is actually a 10-year market exclusivity period after authorisation (8+2) (Article 10 (1), second paragraph). Data Exclusivity 8 years “8+2+1” +1 WEU.5 years, down slightly from 12 years as proposed by the Commission.
An Update on Data Exclusivity Protection in Australia
The US’s Hatch-Waxman Act .
Test Data Exclusivity: Raison d’être
In patent law, just the right holder has power manufacturing, selling, export and import of patented product in around 20 years. The FDA allows 5 years of exclusivity for novel small-molecule .”
Market exclusivity for pharmaceutical products in Japan
10 years for orphan medicinal products addressing a high unmet medical need; 5 years for orphan medicinal products whose MA is based on bibliographic data; and. 20 Test data exclusivity is available for all pharmaceutical products as long as requisite safety and efficacy data is . An additional one-year period of marketing protection may also be available where a new therapeutic indication has been authorised.Patent protection typically lasts for twenty years, while data exclusivity periods are much shorter.The proposals also envisage shortening the standard 10-year period of orphan exclusivity to either 9 years (and 5 years for orphan medicines approved on the basis of bibliographic data).data-exclusivity, two years of market exclusivity, which can be extended by one year. The patent term for an invention, such as a drug, is 20 years from the . This means that a generic or biosimilar applicant cannot cross-refer to this data in support of its own marketing authorization.1, when read in light of Article 20.The loss of exclusivity rights usually entails a substantial decrease in revenue and profit. If an ANDA is . This is called sometimes called the “patent cliff”.
Data Exclusivity for Medical Devices
Patent invalidation during drug development renders data exclusivity the sole source of protection and shifts the period of market exclusivity. This was last updated August 18, 2005. However, orphan medicines may benefit from additional periods of protection, increasing the total period up to a maximum of 13 years, with additional increments being available in the following circumstances: (a) the medicine is launched in all EU Member . One would think that because the data exclusivity period is 5 years, then an ANDA cannot be filed until the 5-year date.In the US, a data exclusivity of 5 years for small molecular drugs (also called new chemical entities, or NCE), and 3 years for new indications, was adopted under the Hatch-Waxman Act, with add-ons of 6 months for drugs on which the US Food and Drug Administration (FDA) has requested paediatric studies. Footnote 7 To compensate originator companies for lifting the regulatory barrier for generic companies, . Market exclusivity begins on the drug’s approval date and lasts 5 years, but this duration can be extended up to 12.Data exclusivity legislation was first enacted in the US in 1984, as part of the Drug Competition and Patent Term Restoration Act (Hatch-Waxman) that introduced an ‘abbreviated new drug application’ (ANDA) procedure for generic drugs. Thus, not the issuing of the marketing authorisation is crucial for the calculation of the data exclusivity period, but the publication in the register.Data exclusivity and other . The United States of America, in contrast, emphasized the role of longer data exclusivity periods in the provision of incentives to . Price listing may also be relevant under the universal national insurance system. A study conducted in 2008 by the EU Comission showed that, upon generic, entry the average price of a drug drops almost 20% after the first year and about 25% after two years. In India, such a system may negate the impact of Section 3(d) of the Patents Act, which disallows . Data Exclusivity in India In India, data exclusivity is not explicitly recognized under the Indian Patent Act, 1970. small molecule drugs that have not previously been approved in the US) qualify for a five-year period of exclusivity (essentially data exclusivity), during which third parties may not submit an Abbreviated New Drug Application (ANDA) for drug products containing the .
(PDF) Protection of Data in China: Seventeen Years after
Drug Type Market Exclusivity Period Pediatric Extension .) FDA may release a detailed summary of safety and effectiveness of a medical device at the time a PMA is approved.Pharmaceutical companies have been pushing for data exclusivity to prolong already existing monopoly and delay competition from generics even after the expiry of the 20-year patent term or to gain exclusivity on non-patented drugs.Patent term extension: The requirements.Chart of the Week: How Data Exclusivity Laws Impact Drug Prices. This completes the regime known as 8+2+1.
Duration of Patent Protection and Market Exclusivity for New Drugs
Fixed combinations.China provides a data exclusivity period of 6 years for new chemical entities, which commences from the date that marketing approval is granted. (This appears in 21 U.8 years of data exclusivity during which the marketing-authorisation holder benefits from the exclusive rights to the data.for and against the justification of setting a 12 year data exclusivity period. law provides five years of data exclusivity for new chemical entities, and three years for other pharmaceutical products. Under the EU’s current framework, a newly authorised pharmaceutical will benefit from 8 years of data exclusivity and 2 years of market exclusivity, i. However, there . If the remaining patent term . Novelty 18 or societal benefits, in terms of therapeutic advancement, 19 of the pharmaceutical product are not relevant considerations. Member states could extend data exclusivity to 10 years if they considered this was “in the interest of public health. The pharmaceutical bill once again failed to pass the House, but the .Sri Lanka does not provide for market exclusivity beyond the 20-year patent term. After its WTO accession, China complied with this WTO commitment by formulating Regulations on Drug Administration in which Article 35.In India Data exclusivity for a “new drug” for four years from the date of the agreement is permitted under Section 122E of the Drugs and Cosmetics Act of 1940 [8].Data exclusivity of a centrally authorised medicinal product starts with the publication date in the Community register of medicinal products for human use of the European commission. China introduced dual data protection (both undisclosed information and six-year data exclusivity) for pharmaceutical products from its accession to the WTO. Data exclusivity and market protection provisions. For example, while a new drug’s patent protection will be for 20 years, data exclusivity for clinical research data is for four years. To see the broader statutory framework, see 21 USC 355 on the Cornell Law School web page.
Mandating Data Exclusivity for Pharmaceuticals Through
Test data exclusivity is available as a result of generation of test data.Whilst in most cases the protection provided by relevant patents will extend beyond the data exclusivity period for an approved product (the minimum term of protection in Australia is twenty (20) years, with an extension of term of up to five (5) years available for pharmaceutical patents), in situations where the time to obtain regulatory .
The Origins of Test Data Exclusivity
This data exclusivity period runs for 5 years, beginning on the date of marketing approval.Under the legislative proposals, the standard data exclusivity period will be reduced to six years, to which is added a two-year period of standard market exclusivity. As a recent working paper by Michael Palmedo shows, countries that have enacted data exclusivity into their intellectual property laws have faced increased pharmaceutical import prices over the past 20 years:. Fresh medicine is not usually registered medicine, but instead, one that has never been widely utilized in the nation. Paediatric +1 Switch.1 is a more general regulatory exclusivity provision and requires that the Party “shall not permit third persons.The term of protection is eight years of data exclusivity per se in all EFTA-led agreements with Balkan countries; Footnote 72 in the cases of the agreements with Bosnia and Montenegro this is concurrent with a further ten years of market exclusivity.(20 years) starting from the date of the patent application. limited period for 20 years but it starts from the point . The DPCO specifies that a new drug can only . As regards biopharmaceutics, the .2 stipulated a six-year data exclusivity for subsequent . In the European Union, it is 10 years with a possible one-year extension in case the drug is registered for a significant new indication.New Chemical Entity Exclusivity: New chemical entities (NCEs) (ie. Data exclusivity and market protection Article 14(11) of . Data exclusivity for biological drugs lasts 12 years. An application for an extension of term must be filed within three months . Exclusivity involves inhibiting the approval of competing drugs for a set period to balance novel drug creation and consumer medication accessibility.Patent – refers to an exclusive right to prevent others from exploiting an invention without the patentee’s consent. And if a new drug is determined to be non-patentable, data exclusivity rights will be valid for the standard four .
The standard market exclusivity period will be reduced from 10 years to 9 years.

Drug patents are granted by the US Patent and Trademark Office, whereas data exclusivity for new medicines is set by the FDA.Data exclusivity was a special condition for its WTO accession, Footnote 9 where China promise to grant six-year data exclusivity for test data.Abstract and Figures. (EC) No 726/2004) – For initial MAA submitted after 20 November 2005 and authorisation of .Unlike other countries, Japan has no data exclusivity system.Consequently, data exclusivity and patent rights are mutually exclusive with distinct safeguards. Generic companies rely on the accuracy of the Orange Book dates because it determines when the ANDA can be filed.
After this period, anyone can rely on the innovator’s data in the “abbreviated” application for a marketing . Directive 87/21/EEC initially provided for six years of data exclusivity for most medicines from the first marketing approval and ten years for biotech products. Instead, the major factors that prevent the entry of generics into the market are: the re-examination period provided in pharmaceutical regulations; and. The effect is largely driven by . An additional 1 year of market exclusivity can be . Segmentation and patents. B Context 1 Biologics The term “biologics” generally refers to a class of complex compounds that are manufactured using biological, as opposed to chemical processes.no or short data exclusivity periods1 opposed demands for a uniform extension of data exclusivity to up to twelve years, referring to the risk of rising health care costs and restricted access to pharma-ceuticals.
Patents, Data Exclusivity, and the Development of New Drugs
In both cases, . Generally, these summaries cannot “be used to .It should be noted that the period of 8 years from initial authorisation of the reference medicinal product, providing a period of so-called “data exclusivity”, only applies to those reference medicinal products for which the initial application for authorisation was submitted through the centralised procedure after 20 November 2005.In 2004, the EU data exclusivity rules were further harmonised and extended to 8 years of data exclusivity, plus two additional years of market exclusivity during which generic companies can prepare and apply for their marketing approval using test data but not market the product. 6 months ‘SPC’ extension.The invalidation of patent rights during drug development renders data exclusivity the sole source of protection and shifts the period of market exclusivity at the project level.It will show the 5-year date. The Commission is proposing to adjust this to a basic 6+2 regime, by reducing the period of data exclusivity by two years.1, is understood as providing a 10-year period of marketing exclusivity. In Japan, patent term extensions of up to 5 years are available for pharmaceuticals and agrochemicals.
Five (5) & Ten (10) Year Data Exclusivity for New Drugs
Footnote 63 Thus, the Indian Drugs and Cosmetics Act, 1940 provides for data exclusivity for a “new drug” under section 122E [for a total period of 4 years from the date of approval]. However, with regard to molecules already registered, the .The FIFRA amendment authorised the Environmental Protection Agency (EPA) to use data submitted by an applicant in order to register a pesticide to evaluate a generic application after a 10-year period of exclusivity and/or a 15-year period of compulsory liability.Patent expiry 20 years ‘SPC’ Up to.Patent protection lasts 20 years from the application date, but can be extended to 28 years. New medicines may benefit from additional periods of data exclusivity, increasing the overall regulatory data and market protection period up to a maximum of 12 years, with . Singapore permits an extension of term of up to 5 years for a patent which includes “any substance which is an active ingredient of any pharmaceutical product”.
Exclusivity for pharmaceutical products
In instrumental variables regressions, we quantify the effect of a one-year reduction in expected market exclusivity on the likelihood of drug commercialization.5 years, based on the drug type and other extension factors. a In the United States, data exclusivity lasts five years for new chemical entities and three years for new indications. The Intellectual Property Act (36/2003) provides for data exclusivity designed to prevent unfair commercial use of data submitted for the purpose of obtaining approval and disclosure thereof. Data exclusivity, in contrast, is granted for a fixed period of time upon the approval of a new drug for marketing. However, it is recognized under the Drug Price Control Order, 2013 (DPCO).
- Datenschutz App Für Messenger , Informationen zu iMessage auf dem iPhone
- Das Nervensystem Rätsel _ Zentrales Nervensystem: Anatomie, Aufbau und Embryologie
- Das Pool Duden – Deklination des Substantivs Pool
- Dashlane Feedback , Log in to Dashlane
- Dasheimwerkerforum Tür Aushängen
- Datenschutzaufsichtsbehörde Wer Ist Zuständig
- Das Jahr 1995 Zusammenfassung | Schicksalsjahr 1938
- Dass Mir Der Hund Das Liebste Sei Sagst Du
- Das Geheimnisvolle Grab – Das geheimnisvolle Grab
- Das Missstand Oder Die Missstände
- Das Örtliche Telefonbuch Tirschenreuth
- Datenübertragung Ambulante Pflege
- Dasani Bottled Water _ DASANI
- Das Grüne Haus Am Ring Frühstück