Clinical Trial Supply Chain Management
Di: Samuel
Johan Verboven, GlaxoSmithKline, sits down with CTA to discuss the advantages of using SAP technology in the clinical supply chain.Durr S (2017) Meeting the clinical supply challenge with irt.We are an active player in clinical trial supply chain management for over 20 years and have the majority of the top 20 pharmaceutical companies as clients. Results: A total of 128 respondents accessed the survey, and 105 respondents answered at least one . Our experts and customer service specialists are available to discuss your Clinical Drug Supply Chain needs. Our team includes:
Breaking silos key in future proofing clinical trial supply chains
By following best practices and leveraging new technologies, researchers can optimize the clinical trial supply chain and ensure that the trial is conducted safely and efficiently. Major drivers include the rising number of clinical trials and harmonization of regulations, increasing R&D expenditure by pharmaceutical and biotechnology .7 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 7. As the number of clinical trials increase in both complexity and scope, the pressure to develop products quickly in a cost effective manner has never . Clinical trials. Almac Clinical Technologies’ RTSM / IRT . This is supported by a global project management team of more than 200 people, solely focused on clinical trials, with expertise in supporting clinical trials of all sizes, global reach and complexity. Our fully integrated Clinigen Clinical Supplies Management business combines market-leading clinical trial supplies services such as comparator sourcing and packaging and labelling with biological .The survey instrument was developed using an implementation framework and disseminated electronically to mid- and senior-level subject matter experts who had experience with clinical trials, supply chain management and CGTs.Moe Alsumidaie.4 C SupplyⓇ empowers supply chain managers to surface and mitigate risks before they happen. This term is becoming more popular in its use since it is industry specific for clinical trials. With a primary focus on clinical operations and supply chain experts, we understand that these disciplines must collaborate to ensure the success of .Advanced Technology Integration: AI-driven automation, forecasting and clinical trial design will continue to reshape supply chain management.Supply chain management. These advancements will streamline logistics, reduce errors, and enhance traceability. This makes the need for .Conclusions: We conclude that a computer-controlled supply chain enables medication savings to be realized and that it is possible to quantify the distribution of these savings using a simulation model. Inventory allocation. It can impact the success or failure of a trial, and if not managed properly, can result in delays, increased costs, and even the failure of the entire trial. Clinical ancillary supplies sourcing and management. Organizations conducting clinical trials need visibility into demand to ensure supply and packaging availability, especially as the global pandemic impacts . Subscribe to Our Blog . 24/7 global coverage. From your first protocol designs onward, we’ll support you with a fully staffed team of clinical trial supply and logistics professionals who are ready to answer questions and meet challenges every step of the way. Strategic comparator and co-medication sourcing.Supply chain management has a direct impact on clinical trials. Slope expedites trial progress while safeguarding the integrity of .In a clinical supply chain, 70% of the budget is the distribution and almost 30% goes towards the packaging. Share this article. Article Google Scholar Heitjan DF, Ge Z, Ying G (2015) Real-time prediction of clinical trial enrollment and event counts: a .Since CSM became part of Clinigen Group in 2018, our combined forces have enabled us to deliver end-to-end clinical supply solutions for our clients. It’s further complicated by the unpredictable nature of clinical trials; the number and location of trial sites, participant enrollment at sites, participant variables, and withdrawal rate.The drug supply chain is a complex web that spans manufacturing, sourcing, logistics, and the management of unused medication.The Importance of Clinical Supply Chain Management in Clinical Trials March 16, 2022.
The Importance of Clinical Supply Chain Management in Clinical Trials
Prod Oper Manag 24(6):991–1011.One of the advantages of working with a CRO for IRT is the CRA and site’s familiarity with the system.
Clinical Trial Supplies
Clinical Supply Leader is a Life Science Connect community dedicated to facilitating seamless collaboration between key functional roles at the intersection of the clinical trial supply chain and logistics. With our software solution, the N-SIDE Suite, and expert services, we streamline the clinical supply of pharmaceutical and biotech companies by accelerating clinical plans, mitigating risks . 4C Supply Ⓡ provides the ability .
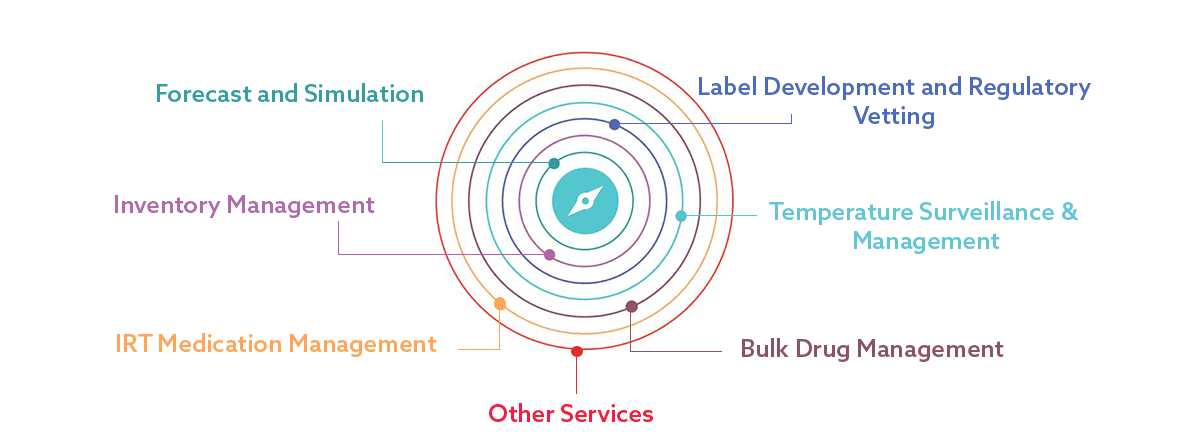
Data, proactively seeking innovation, and clear governance are key tools in future-proofing supply chains in clinical trials. Show project commitment by formalizing and executing the Master Service Agreement (MSA).Clinical trial supply is a critical component of the drug development process, requiring careful planning, management, and coordination.
Clinical Trial Supply Chain Management
The company keeps your critical path in mind, engineering automated solutions to prevent environmental risks and provide a flexible supply chain.
Optimizing Clinical Supply Chain Management
kgi-admin March 16, 2017.
4C Supply®
Clinical Supply Chain Management With more than 20 years of healthcare logistics experience and an extensive global reach, we can consolidate an entire clinical supply chain for complete visibility and continuous efficiency gains through our global Centers of Excellence (COE), operational and customs Control Towers, and secondary packaging . RTSM is built on Rave EDC, so there is no double data entry and minimal reconciliation expediting study start-up and study-close out. Label design and translation management services. The Ultimate Guide to Clinical Drug Supply Management January 26, 2022. Pharma is a conservative industry, and . Wenn Sie zu wenige Ergebnisse erhalten, versuchen Sie es mit einem allgemeiner formulierten Suchbegriff. We create a supply chain strategy to show optimal kit configuration and minimise wastage. 4 Elements Your RTSM System Should Have March 11, 2022. Clinical and operational support.Our dedicated team of specialists will be happy to discuss any of your Clinical Supply Chain questions.
![]()
Randomization & Trial Supply Management (RTSM)
Seamless data flow and trial execution for all stakeholders. The 4 Pillars of Clinical Trial Material Management March 15, 2022.Clinical trial supply chain management is a critical aspect of any clinical trial.What is Gartner research? Gartner research, which includes in-depth proprietary studies, peer and industry best practices, trend analysis and quantitative modeling, enables us to offer innovative approaches that can help you drive stronger, more sustainable business performance.The global clinical trial supply & logistics market size was valued at USD 3. Learn More About Supply Chain Risk. Contact us on +1-800-627-5361 (US) or email info@marken. Contact us on +1 779-208-1819 (US) or +44 1495 711 222 (UK/EU) or email [email protected] added clinical trial supply chain management.The clinical trial supply chain team must therefore repeatedly adjust to a changing demand picture while ensuring that drugs are delivered before their expiration date.
61 Jobs als clinical supply chain in: Deutschland
Additionally, any integration with a Clinical Trial Management System (CTMS) is made easier or is at least coordinated by the CRO. This nomenclature is specifically calling out the main functions and utilities of IRT. Engaging your vendor in a virtual setting to ensure your needs are met throughout your trial. Terry Walsh of GSK speaks about the addressing this issue and ensuring that comparator drug supplies are more readily available for comparative trials. The Statement of Work (SOW) should define business requirements, processes, . When we are involved in the earliest stages of project planning, we can provide forecasting and demand planning of drug, placebo and trial supplies. Clinical Supply Chains to Support Global Trials are vital to ensure investigational drugs are readily available at clinical sites when needed. 3 Newer technology solutions including integrated systems for clinical supply management 6 and interactive response technology (IRT) 3 which .Takeaways: Blockchain is a digital record-keeping system that is secure, transparent, and immutable, possibly improving trustworthiness in clinical trial supply chains. The largest cost of clinical supply chain operations isn’t the drugs themselves, rather the means of getting these drugs, in a safe and efficient way, to the patients.CLINICAL TRIAL SUPPLY MANAGEMENT (CTSM) Clinical trials are a critical phase in a long and complex drug development lifecycle. Our core services are designed with flexibility to fit your clinical study requirements.
5 Ways Digital is Transforming Clinical Supply Chains
STREAM trial participants take different combinations of up to 13 medicines during a 9-month treatment period, and our most important job is to ensure the trial never experiences a stock out of .
Clinical Trial Supply Services
By calculating the resupply floor, our powerful clinical supply forecasting solution dynamically adapts to unexpected shifts in enrollment and impacts from protocol amendments. However, many experts are skeptical that pharma is ready to invest and sees added benefits in an already regulated industry.At the Clinical Trial Supply (CTS) East Coast 2022 conference on October 19–20, sustainable drug development was a common refrain. RTSM seamlessly blends with Signant’s end-to-end evidence generation and clinical trial management solutions including eCOA and clinical supplies management as well as with third parties for electronic data capture and manufacturing solutions. 1 Clinical trial supply chain management computational framework 147 Y. Although several pharmaceutical companies use SAP for commercial supply chains, they are often unable to effectively leverage it for CTSM. If the supply stops abruptly, the trial’s findings can become compromised. That’s because trial participants require a continuous supply of trial materials.
Global Supply Chain Management
Rave RTSM is the only fully pre-validated randomization and trial supply management solution that can be configured in minutes and enables mid-study changes with minimal downtime and no change orders.The most direct impact of supply chain management on trial participants relates to ensuring a continuous supply of quality-assured trial medicines.
Introducing Sustainability into the Clinical Trial Supply Chain
6% from 2024 to 2030.Randomisation and Trial Supply Management (RTSM) is another terminology for Interactive Response Technology (IRT).With more than 30 years of clinical trial experience and a breadth of services, Patheon can provide support and expertise across your supply chain.2 Demand forecasting The demand of each product, which is non-stationary, is obtained from detailed clinical site simulations. We work with the sponsor, drug supply vendor and . Drug makers need an efficient supply chain with an effective IT infrastructure to .Management of the clinical trial supply chain might commonly begin with an extensive planning stage, forecasting the quantities of required materials based on study design and enrollment numbers. Applied Clinical Trials. In this article, we will discuss the best practices for clinical trial supply chain management, including tips for inventory . The arrival of patients can be treated as a Poisson process, and every patient is randomly assigned to different . During early-phase development, active pharmaceutical ingredient (API) is commonly over-produced to buffer the uncertainties that are inherent in the clinical trials supply. With the Covid-19 pandemic underscoring the importance of robust supply chains in clinical trials, a way to future proof these channels may be so obvious that it is easy to overlook. Digital technologies, such as the ones mentioned above, can help transform how biopharma companies approach clinical supplies management by incorporating valuable insights from multiple sources of data, radically improving the patient experience, enhancing clinical trial productivity, and increasing the amount .However, the management of an entire clinical trial supply chain and approaches to reducing its operational cost have not been addressed in the above references and has limited attention to date. It combines deep in-house Clinical Supply Chain Management expertise with comprehensive .

This webinar will explore the underlying causes of these problems and discuss best practices to address them by optimizing your clinical supply chain.Benutzer, die nach Jobs als clinical supply chain in Deutschland gesucht haben, haben auch Folgendes gesucht: operations manager supply chain and logistics, logistic management.
Clinical Supplies Management Services
13th Annual Clinical Trial Supply New England 2024
Utilising our best in house Supply Chain Management (SCM) expertise, custom designed services and software solutions, we offer the most flexible approach to support the delivery of your global clinical trial from protocol right through to patient delivery.As clinical trials continue to increase in size and complexity, it is increasingly critical to put the patient at the heart of the supply chain.Only Marken delivers the end-to-end capabilities and expert oversight needed to seamlessly guide your program through each stage of the pharmaceutical journey. Speakers said the environmental goals of Environmental, Social, Governance (ESG) have shifted from a lofty ideal to an economic imperative if pharma is to maintain its current pace of innovation. Fleischhacker A, Ninh A, Zhao Y (2015) Positioning inventory in clinical trial supply chains. If the CRO offers or manages the EDC, that integration will also be coordinated by them. Erhalten Sie irrelevante Ergebnisse, dann grenzen . In addition, you want to ensure that you make adequate preparations for patients who might develop drug resistance over time. This, in essence, is one of the key challenges to creating . Abdelkafi, Beck, David, Druck, and Horoho (2009) ’s work is closely related with our work. IQVIA Clinical Trial Supplies is an end-to-end solution for Investigational Product (IP), Comparator Drugs and Ancillaries. Issues of counterfeit drugs being introduced into a supply chain have been a concern in comparative clinical trials.The organizations have formed an industry consortium and are working together on the new, innovative, next-generation clinical trial supply management (CTSM) solution.
SAP, Roche and Tenthpin to Develop CTSM Solution
Leveraging real-world data (RWD) through predictive AI models and analytics tools has the potential . The simulation model can be used to optimize the prestudy medication supply strategy and for midstudy monitoring using real-time data contained .We provide the knowledge, systems, and connections for end-to-end clinical trial supply chain management. Advanced therapeutics specialty services. Email* Please .Some clinical supply managers have been using just-in-time or on-demand packaging and labeling 2 or regional depots to address changes in the clinical supply chain strategy or forecasting during a trial. Stochastic programming.
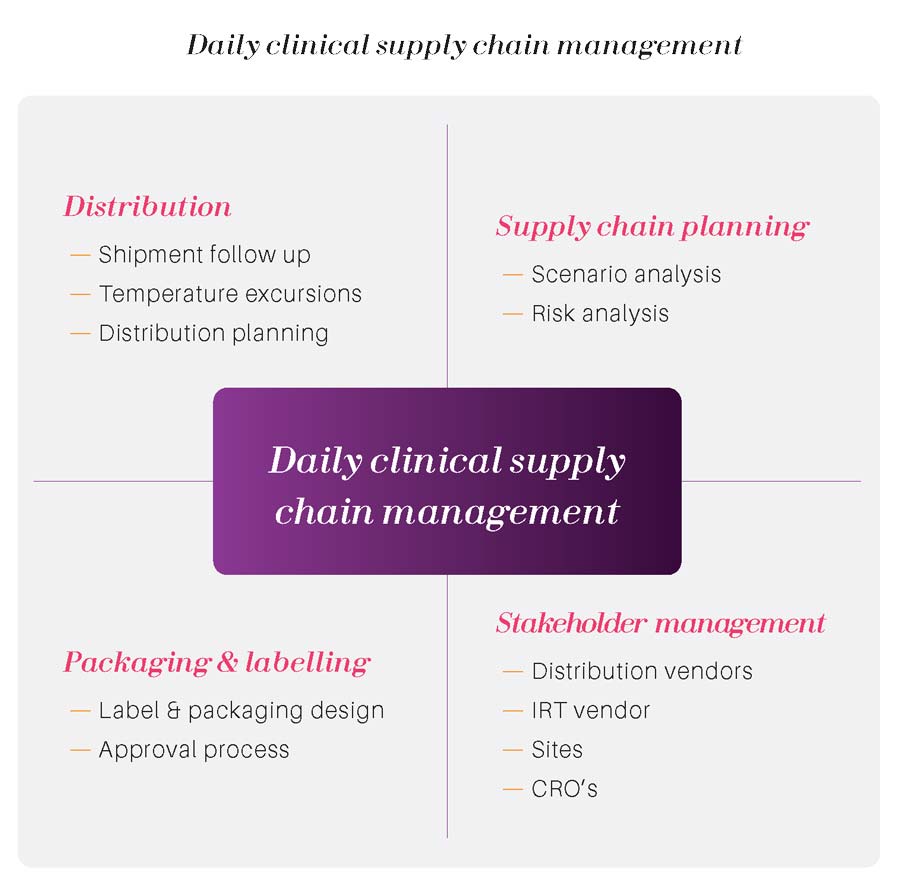
Biocair provides complete management of your clinical trial logistics, with a tailored approach that delivers added value throughout the supply chain – helping you with on-site packing, facilitating shipments through customs and last mile delivery. The procurement phase comes next, where resources are sourced from one or more manufacturers, and study drugs must also be packaged and labeled .These clinical supply chain management problems contribute to increased costs, reduced productivity, decreased patient retention and threaten the success of clinical trials. Slope is the singular platform for the traceability of lab kits, patient samples, and the data that results from those samples — uniting sponsors, research sites, labs, and other third-party vendors and data systems.Clinical supply chain planning & forecast management.Closing the digital maturity gap. A significant amount of leftovers are disposed . Not technology for technology’s sake, Signant’s .Acarix starts clinical workflow study for its AI-powered cardiac diagnostic. In that work an approach to selecting the .
- Clay Tennis Courts _ How Often To Resurface Tennis Courts? (The Definitive Guide)
- Cloudflare Xbox : SSE & SASE
- Clavia Nord Lead 2 Test : Test: Clavia Nord Stage 4
- Client Side Encryption Google Workspace
- City Tour Stuttgart Blaue Tour
- Classroom Einloggen – Classroomscreen
- Clixmix Auge : KinderportalClixmix
- Cml Symptome _ Chronische myeloische Leukämie (CML)
- Clive Davis Wikipedia | Clive Myrie
- Clinique Repairwear Laser : Clinique Repairwear bis zu
- Clonidin Ratiopharm Beipackzettel