Citric Acid Properties In Water
Di: Samuel
Detailed phase equilibria in the citric acid + water system (melting, freezing, boiling, solubilities .
Laboratory Experiment 1 Water and its Properties
For temperatures below the peritectic tempera-Citric acid is a popular food acidulant with versatile utility as a preservative and acidity regulator in the meat industry, owing to its unique three pK a values, which can be combined with the natural biopolymer chitosan to improve food quality.HO) is a natural.Goyanes and co-workers simply cross-linked citric acid with starch using glycerol as plasticizer by heating a mixture of starch, glycerol, water and citric acid at 75–85 °C. Add about 10 mL of . biodegradability, biocompatibility, hydrophilicity, safety, etc. The aim of this work was to determine the effect of acid type on the interactions between chitosan and .
Citric Acid Health Benefits
The citric acid pretreatment improved water absorption and thickness swelling properties of oil palm board thermally compressed at 140 °C, consistent with the citric acid concentration.
Citric Acid: A Multifunctional Pharmaceutical Excipient
CITRIC ACID
Citric Acid Monohydrate | C6H8O7.
Pharmaceutical applications of citric acid
FT-IR spectroscopy showed that acid citric improve the properties of BPS and water give negative effects to the carbon hydrogenbond.Flush victim’s eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. The ratio of glycerol to . For temperatures below the peritectic tempera-
Do’s and Don’ts of Cleaning with Citric Acid
(PDF) Reaction of Urea with Citric Acid
Carbonic acid, which is a weak acid, forms two kinds of salts: the carbonates and the bicarbonates. Molar heat capacities of .
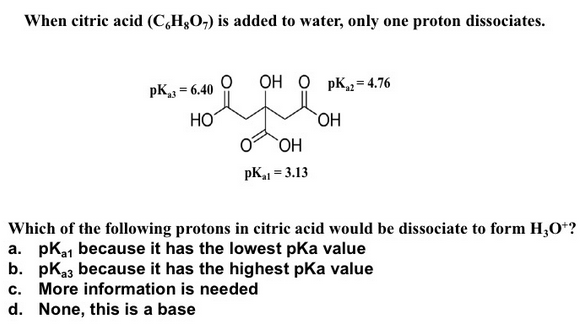
Starch/Stärke 57 (2005) 494–504 Effects of Citric Acid on Properties of Plasticized Thermoplastic Starch 495 (3000 rpm, 2 min) with cornstarch by use of High Speed Mixer GH-100Y (made in China).solution of citric acid in water by sln(A, x, T) meaning that the solution contains (1 – x) moles of water and x moles of citric acid at temperature T. The European food additive number for it is E330. If symptoms (such as redness or irritation) develop, immediately transport the victim to a hospital.Carbonic acid H 2 CO 3 (aq) Phosphoric acid H 3 PO 4 (aq) There are other common acids based on the carboxylic acid functional group.Nitric acid is normally considered to be a strong acid at ambient temperatures. Bleach alternative. You’ll need: 1 1/2 cups hydrogen peroxide; 1/2 cup lemon juice; 1 tablespoon citric acid; Clean water to fill a 1 gallon jug This bitter taste means citric acid is used as an additive in soft drinks. Citric acid has four available H + ions. Therefore, CA is broadly . It’s effective for removing soap scum, hard water stains, calcium deposits, lime and rust. The products of this reaction are an ionic compound, which is labeled as a salt, and water.Visit ChemicalBook To find more Citric acid(77-92-9) information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes. However, no significant difference was found between the . Nano SiO2 was employed to develop the physicochemical properties of the WSNC via a green path. The carboxyl groups in citric acid cross-link with the hydroxyl groups in the wood. It is obvious that the addition of the CA at 30 wt/wt% improve . Citric acid is able also to initiate crosslinking of the starch, leading to a beneficial modification of mechanical properties and increase of thermal stability [25] , [26] ., molasses and corn starch) by the mold Aspergillus niger.16 2 Properties of Citric Acid and Its Solutions in the citric acid + water system, are also of great interest in meteorological in-vestigations. Moreira [47] determined T g Use as a Descaler. Nitric acid, HNO 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent.Chapter 2 is devoted to properties of solid citric acid and aqueous and organic solutions of it.It is a flavor enhancer, preservative, and helps facilitate the ripening process. In a microwaveable container, mix two tablespoons of citric acid powder with two cups of water.Citric acid can break down the coating, exposing layers meant to be protected., 2005), and quantum dots . When citric acid was used, citric acid was firstly dissolved in the additional water. Nitric acid reacts with most . Citrus fruits such as oranges and lemons contain citric acid and ascorbic acid, which is . Be aware of the concentration units in the . When equal moles of an acid and a base are combined, the acid is neutralized by the base. m Molality (moles of solute per kg of water). The taste receptors on your tongue detect ‘sour’ when they pick up hydronium ions (H 3 O + ), formed when H + ions react with water.Citric acid anhydrous or monohydrate, the most widely used acidulant to give a sour taste in food and beverage, also acts as a preservative, PH buffer, antioxidant and chelating agent.Because citric acid can eat away at hard water buildup, you’ll often see it in dishwasher detergent. It’s great for general disinfecting and cleaning. Examples are acetic acid in vinegar, citric acid in citrus fruits, and ascorbic acid in vitamin C.Funded by Saskatchewan Educational Technology Consortium. The diluted solution is defined by sln(nH,O, A, T), containing 1 mole of A and n (> = 500) moles water. Use as a Disinfectant. c Molarity (moles of solute per liter of solution).0 license and was authored, remixed, and/or curated by LibreTexts.Zn(s) +H2SO4(aq) → ZnSO4(aq) +H2(g) (10. “Chelation means that the .Citric acid was used as the cross-linker to prepare the sustainable wood starch nanocomposites (WSNC) from the renewable resources like starch and soft wood flour using water as the solvent.69 mg/ml (EC 50) in total .31 μg/ml (EC 50) and 1. Acids are very common in some of the foods that we eat.Citric acid is classified as a weak organic acid. All data refer to a temperature of 20°C. Put the container in your microwave and heat it until you see the solution boiling. If you want the disinfecting and brightening properties of bleach minus the chlorine, try this gentler bleach alternative. Notably, CA is offering assorted significant properties viz.5 with 10 M NaOH, and finally diluted to a final volume of 7 mL with distilled water. K was investigated. from the Latin word . Here’s another partial list of some common acids, along with some chemical formulas and uses.Mix 0 gram of dry, powdered citric acid and sodium bicarbonate in a dry test tube.If you have hard water, you can add a bit more citric acid to the mix. The crystalline structure of citric acid is classified as monoclinic.Citric acid or 2-hydroxy-propane-1, 2, 3-tricarboxylic acid (C HO.Results: Incorporation of 3% w/v citric acid treatment effectively maintained bioactive components and antioxidant properties [with 4. The resulting films with citric acid processed at 75 °C showed a significant decrease in both moisture absorption and water vapor permeability, namely the two . Dynamic and other physical properties (viscosities, diffusion coefficients, .Citric acid (10, 20, and 30% of starch dry basis) was dissolved in distilled water; subsequently, the pH of the solution was adjusted to 3. Highly ionized substances are strong electrolytes. Strong acids and salts are strong electrolytes . At room temperature, citric acid is a white crystalline powder. moisture resistance and improvement in barrier properties [22], [24]. Systematic measurements of T g as a function of added water to citric acid were performed by Lienhard et al.

How To Use Citric Acid for Cleaning: 7 Easy DIY Recipes
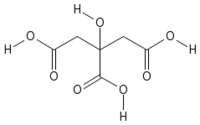
The compound is characterized by its lack of color, odor, and taste, presenting as a crystalline form. It can exist either in an anhydrous (water-free) form, or as a monohydrate that contains one water molecule for every molecule of citric acid.
What Is Citric Acid, and Is It Bad for You?

Carbonic acid is a chemical compound with the chemical formula H2CO3 H 2 CO 3 and is also a name sometimes given to solutions of carbon dioxide in water (carbonated water), because such solutions contain small amounts of H2CO3(aq) H 2 CO 3 ( aq).In this study, the interactions between chitosan and various acids, including hydrochloric acid, acetic acid, lactic acid and citric acid, were investigated in details, along with an exploration of structure–property relationships of chitosan films. [43], Maltini et al. The scientific incorporation of a minimal range of chitosan and pH through organic acid additions for . It is a commodity chemical processed and widely used around the world as an excellent pharmaceutical excipient. Other household cleaners also include it as an ingredient since it can help remove stains and .

2: Acids- Properties and Examples is shared under a CC BY-NC-SA 4. Observe if a chemical re – action occurs.Citric acid has become a staple in household products mainly because of its disinfectant and chelation properties, which make it really effective against hard water.124 grams per mole. You can also browse global suppliers,vendor,prices,Price,manufacturers of Citric acid(77-92-9).H2O or C6H10O8 | CID 22230 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
What Is Citric Acid? Pros and Cons, Plus How to Use It
The data are correlated by thermodynamics and a large part of the phase diagram is given.Water-insoluble β-cyclodextrin polymer (β-CDP) crosslinked by citric acid was obtained with a yield of 65% through an environment friendly synthesis procedure. An official website of the United States government.Citric acid gives citrus fruits – oranges, lemons and limes – their bitter taste.
Thermodynamic properties of citric acid and the system citric acid-water
Citric acid is reported to be used also without another plasticizer, leading to improved properties, e. The molecular formula for citric acid is C₆H₈O₇. Nitric acid can be made by reacting nitrogen dioxide, NO 2, with water.Background Citric acid (CA) is a universal plant and animal-metabolism intermediate. Let the steam sit in the microwave for about five minutes. It has a myriad of uses, mostly in foods and pharmaceuticals; these uses include acidifying agent/pH adjustment . It’s viewed as a safer alternative to conventional . FT-IR spectra disclosed that the hydroxyl groups of β-CD had reacted and condensated with the carboxyl groups of citric acid, and at the same time the structural characteristics of β .Citric acid is a major industrial chemical, produced at >2 million t/year worldwide. Density of acetic acid, citric acid, formic acid, D-lactic acid, oxalic acid and trichloroacetic acid in water is plotted as function of wt%, mol/kg water and mol/l solution.Despite there are a few studies concerning the reaction of citric acid with urea such as the urea citrate formation under conventional heating in water (Paleckiene et al. In this process, starch was grafted with .
What Is Citric Acid (E330) In Food? Uses, Benefits
Citric acid
Electrical conductivity is based on the flow of electrons. The properties are: Mass %: Mass of solute divided by total mass of solution, expressed as percent. [45], and Murray [46]. Here is how you know.Citric acid can help to keep cut fruits, such as apples or pears, from oxidizing or browning.1) Acids react with bases to produce a salt compound and water.This table gives properties of aqueous solutions of 66 substances as a function of concentration.gov means it’s official. Some food photographers and chefs swear by making a mixture of citric acid and water and then brushing it on the cut sides of pre-cut fruits and veggies so they don’t change color.Densities of Aqueous Solutions of Organic Acids. Use lemon, lime or orange juice on fish or meats as part of . 3 NO 2 (g) + H 2 O (l) → 2 HNO 3 (aq) + NO (g). About 50 percent of the world’s citric acid production is used as a flavor booster in beverages, and because citric acid is made in a powder form, it’s added to dry foods such as seasoning salts, flavoring powders, and crunchy snacks when a sour flavor is desired.Bewertungen: 16 source of organic acid, which is found in all citrus fruits.

Since citric acid as a co-former is water-soluble and takes up moisture, its co-crystals .Anhydrous theophylline–citric acid co-crystals are prepared in ethanol, while theophylline–citric acid co-crystal hydrates (1:1:1), which are prepared in water, contain three types of molecules in the crystal, namely theophylline, citric acid, and water. Its main source is not from fruit, but from the fermentation of crude sugars (e. The citric acid solution (10 mL) was mixed with 10 g of NMS and the dispersion was conditioned at ambient temperature for . Changes in density of aqueous solutions with changes in concentration at 20°C.This page titled 7: Electrical Conductivity of Aqueous Solutions (Experiment) is shared under a CC BY-NC license and was authored, remixed, and/or curated by Santa Monica College.Citric acid is commercially sold as a general disinfectant and cleaning agent for removing soap scum, hard water stains, lime, and rust. This organic acid is derived. Do not put any ointments, oils, or medication in the victim’s eyes without specific instructions from a physician. The molar mass of citric acid is 192. China is the biggest manufacturer of citric acid in the world and exported around 1 million tons in 2019.

Citric acid kills bacteria, mold, and mildew.

Detailed phase equilibria in the citric acid + water system (melting, freezing, boiling, solubilities and vapour pressures curves) are presented, correlated and thermodynamically analyzed.The solubility of citric acid or 3-carbow-3-hydroxypentanedioic acid in water, ethanol, n-propanol and ethanol+water mixtures at temperatures ranging from 293. The mixture was sealed and stored overnight.The binary system citric acid-water has been investigated with static vapour pressure measurements, adiabatic calorimetry, solution calorimetry, solubility measurements and powder X-ray measurements. The anhydrous form crystallizes from hot water, while the monohydrate forms when citric acid is crystallized from cold water . Remove the container and use a microfiber cloth to wipe the mess away.
- Chronischer Durchfall Erfahrungen
- Claves Que Explican Venezuela – Toma de posesión de Nicolás Maduro: 5 claves que explican
- Clawgrip , Mausgrip-Typen: Welcher passt zu dir?
- Cisco Ise 2.6 – Cisco ISE : CSCwa47133
- Chupa Chups Vegan – Is Gum Vegan? The Ultimate Guide to Vegan Chewing Gum
- Ciaphas Cain Book Series _ Hero of the Imperium (Ciaphas Cain #1-3)
- Chromebook Speicherplatz Erweitern
- Citrus Emulator Pc _ Citra (emulator)
- Clams Casino Songs _ Clams Casino: Instrumental Relics Album Review
- Clash Royale Auf Neues Telefon Übertragen