Carbon 14 Atoms – Half-Life Calculator
Di: Samuel
Carbon-12 is the most abundant, with a natural abundance of 98. If a sample of a tree (for example) contains 64 grams (g) of radioactive carbon after 5,730 years it will contain 32 g, after another 5,730 . Im Periodensystem steht es in der vierten Hauptgruppe bzw.
Carbon-14 Atom
Carbon C-14 is an unstable isotope of carbon created when a neutron collides with a nitrogen atom, causing capture of the neutron and release of the proton converting nitrogen to a carbon with fourteen nucleons (6 protons and 8 neutrons). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Simplified schematic layout of an accelerator mass spectrometer used for counting carbon isotopes for carbon dating. One of the most well-known applications of half-life is carbon-14 dating. Since carbon 14 is a radioactive isotopes of carbon, it is not stable (meaning it does not last forever without turning into something else).
Carbon
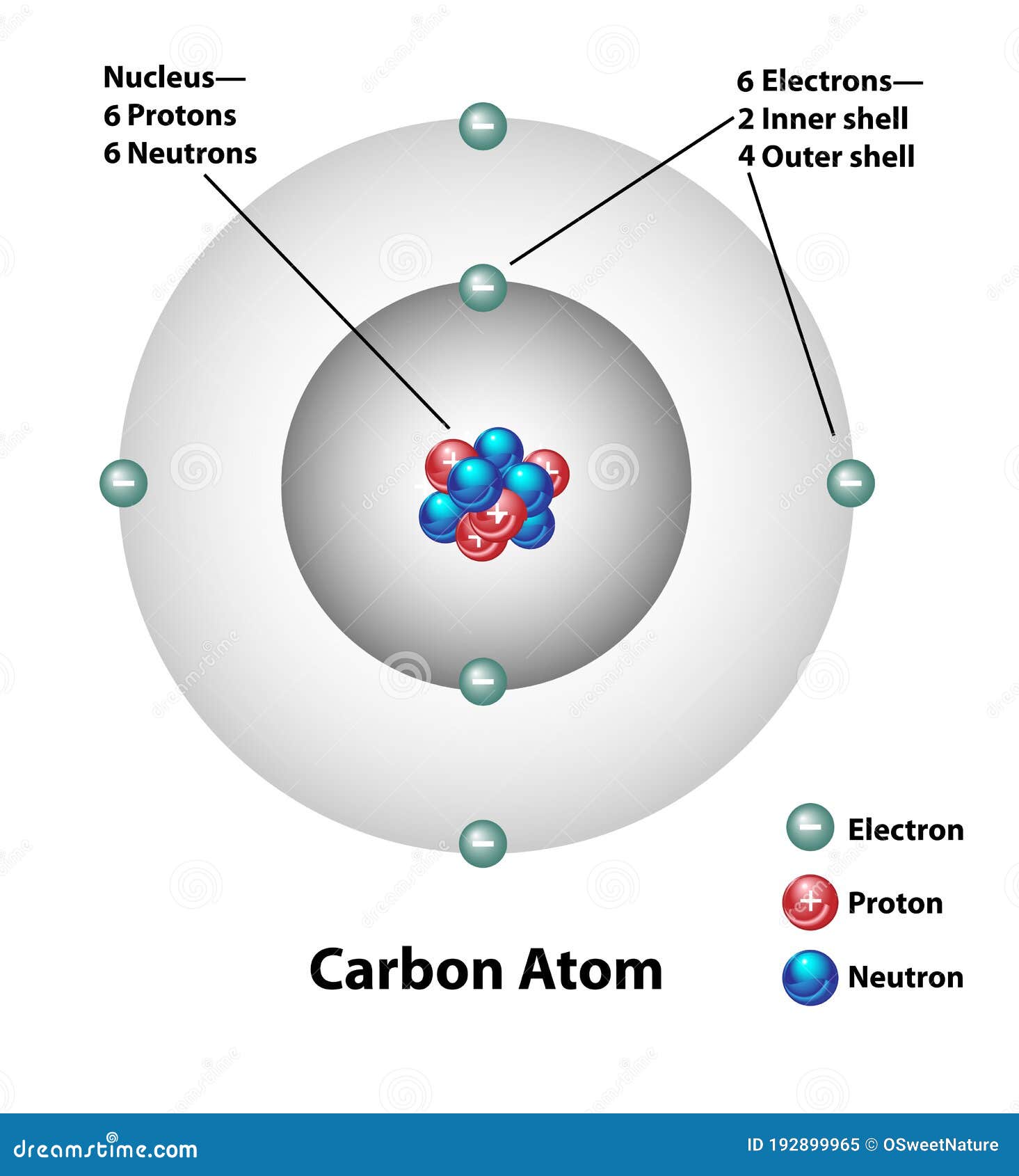
Carbon is a chemical element with atomic number 6 which means there are 6 protons in its nucleus.For example, a carbon atom weighs less than 2 \(\times\) 10 −23 g, and an electron has a charge of less than 2 \(\times\) 10 −19 C (coulomb). These atoms are the isotope called . Both processes of formation and decay of carbon-14 are shown in Figure 1.Carbon-14, or carbon atoms with eight neutrons in their nuclei, is unstable, and is so rare that only one-in-a-trillion carbon atoms are carbon-14. Applications of radiocarbon dating are remarkably diverse.Carbon-14 occurs rarely in nature.The total electrical charge of the nucleus is therefore +Ze, where e (elementary . The sample, often in the form of graphite, is made to emit C .Allgemeine Daten. With a half-life of 5,700 ± 30 years, detection .02214076×10 23 is known as the Avogadro’s number. Radioactive carbon has the same chemistry as stable carbon, so it combines with the ecosphere and eventually becomes part of every living organism. Archaeologist of course often need to find how old their artefacts are, and therefore also .Atomic mass of Carbon is 12.
Carbon Dating
Flexi Says: The main difference between carbon-12 and carbon-14 atoms is the number of neutrons in their nuclei.
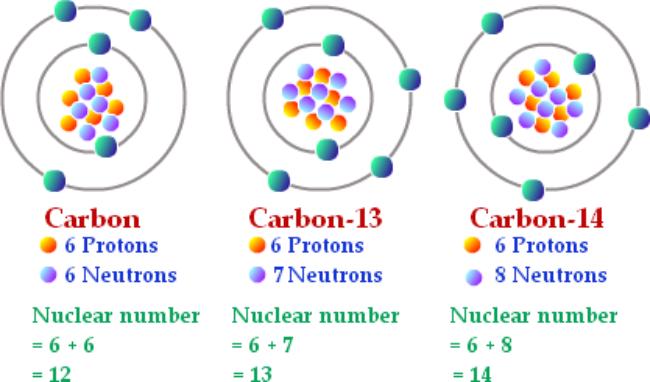
A tiny percentage of a living thing contains carbon-14 atoms. The whole story doesn’t come together until you include the three .Principe de la datation au carbone 14.Alkanes with five or more carbon atoms are named by adding the suffix -ane to the appropriate numerical multiplier, except the terminal -a is removed from the basic numerical term. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for . Both have 6 protons, which is what makes them carbon atoms.
The Cosmic Story of Carbon-14
If we can measure the ratio of carbon-14 atoms to the total amount of carbon, we can use the radioactive decay law to compute how long that object has been out of the carbon cycle, thereby “dating” it. carbō,Holzkohle‘, latinisiert Carboneum oder Carbonium) ist ein chemisches Element mit dem Elementsymbol C und der Ordnungszahl 6. Carbon-12 has 6 neutrons, while carbon-14 has 8 neutrons.Carbon-14 can also be produced in the atmosphere by other neutron reactions, including in particular 13C(n,γ)14C and 17O(n,α)14C. In a typical natural sample, about one in a trillion (1 / 10 12) of these will be carbon-14, giving us about 50 billion (5 × 10 10) carbon-14 atoms in each gram of carbon. When living thing dies it no longer takes in new carbon atoms. The amu was originally defined .Die Radiokarbonmethode, auch Radiokohlenstoffdatierung, 14 C, C14-Datierung oder Radiokarbondatierung bzw. The uncertainty in this dating is generally . kul-a-, kul-ō(n)-,Kohle‘) oder Carbon (von lat. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or . A partir du moment où ces organismes meurent, le carbone n’est plus .Most carbon atoms weigh 12 atomic mass units.
Radiocarbon Dating Calculator (Carbon 14 Dating)
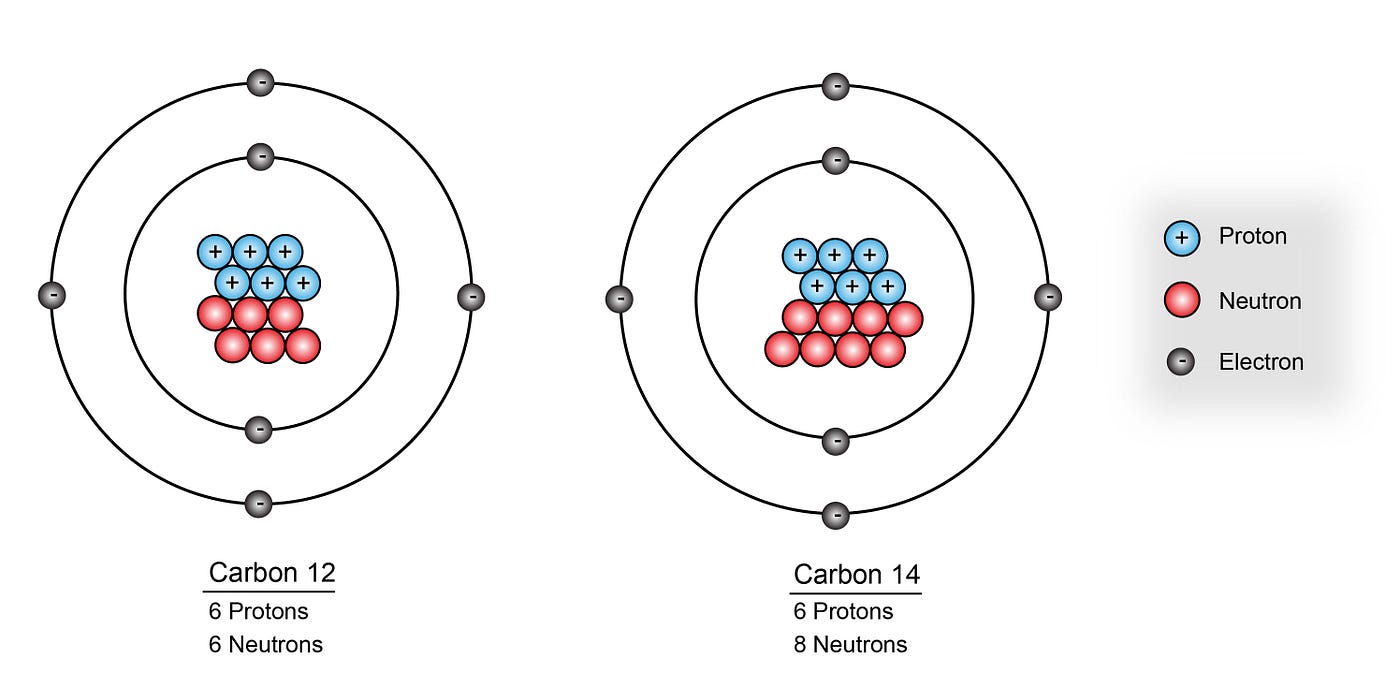
Das ist bis zu einem Alter von 50 000 bis 60 000 Jahren möglich, bei noch älteren Funden sind schon so viele Atome zerfallen, dass der 14 C-Gehalt nicht mehr zuverlässig nachweisbar ist. But the carbon-14 it has already assimilated continues decaying back to 14 N with a half-life of 5,730 years.For most elements other than hydrogen, isotopes are named for their mass number. Living organisms use atmospheric carbon dioxide, whether with stable or radioactive carbon, through processes of photosynthesis and respiration, and thus their . Carbon-14 is also called “carbon 14” or “radiocarbon”, or in symbols: 14 C, or 14 6 C, or C 14. Bezeichnung des Isotops: Kohlenstoff-14, C-14 Namen: Radiokohlenstoff, Radiocarbon Englische Bezeichnung: Carbon-14 Symbol: 14 C Massenzahl A: 14 Kernladungszahl Z: 6 (= Anzahl der Protonen) Neutronenzahl N: 8 Isotopenmasse: 14,003241988 (4) u ( Atommasse) Nuklidmasse: 13,9999505 u . Carbon-12 and carbon-13 are stable, while carbon-14 is radioactive, with a half-life of around . How electrons are arranged The electrons in an atom circle fast around the nucleus, at different levels from it. IUPAC-Gruppe oder Kohlenstoffgruppe sowie der zweiten Periode. Se formant dans la haute atmosphère de la Terre, il existe 1 atome de carbone 14 pour 1 000 milliards de carbone 12 (isotope non radioactif). Carbon 14 dating uses the measurement of the ratio of carbon 14, out of all carbon atoms, within something.The term is most commonly used in relation to atoms undergoing radioactive decay, but can be used to describe other types of decay, whether exponential or not. Early physicists assigned the atomic mass of 12 to the carbon-12 isotope (which is the most common carbon isotope) so that it would be easier to determine the atomic masses of other atoms.Le principe de la datation. Carbon-14 has an abundance of 1.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Sa période radioactive, temps au bout duquel la moitié de ces atomes s’est désintégrée en azote 14, est de 5 730 ans. For example, carbon atoms with the usual 6 neutrons have a mass number of 12 (6 protons + 6 neutrons = 12), so they are called carbon-12.
More than 5000 samples analysed at the Tandem Laboratory
On average just one out of every 1.
Carbon 14
There are three naturally occurring isotopes of carbon, 12 C and 13 C (stable isotopes), while 14 C is a radionuclide, that have a half-life of about 5,730 years.
Der zeitliche Anwendungsbereich liegt etwa zwischen vor 300 und etwa 60. It is also called radio carbon because it is radio active (but not dangerous).Hot Carbon: Carbon-14 and a Revolution in Science John F.
Physicists explain the long, useful lifetime of carbon-14
Marra Columbia University Press (2019) It is nearly 80 years since the discovery of carbon-14, a radioactive isotope of the sixth element.
Atoms to Moles Calculator
Radioactive dating is a process by which the approximate age of an object is determined through the use of certain radioactive nuclides.
More generally, The atomic mass is the mass of an atom.72 × 10^24 (1mole/6.Atomic Number – Protons, Electrons and Neutrons in Carbon. The +2 oxidation state is also seen in compounds such as carbon monoxide. However, to determine the initial number of carbon-14 atoms in the organism at death, we need to make some assumptions and calculations.Carbon-14, however, . When a plant/animal dies, it stops absorbing new carbon-14 atoms. 6 is the Atomic number for all carbon isotopes.022140857 x 10^23 )= 7. This difference in neutron number makes carbon-14 a radioactive isotope of carbon, while carbon-12 is stable. As a result, carbon-14 is continuously formed in the upper atmosphere by the interaction of cosmic rays with atmospheric nitrogen. It has an atomic nucleus containing 8 neutrons and 6 protons.Carbon usually has a valence of +4, which means each carbon atom can form covalent bonds with four other atoms. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Half of it will decay in about 5,730 years to form nitrogen. The radioactivity of carbon-14 provides a method for dating objects that were a part of a living organism. Accelerator techniques for carbon dating have extended its range back to about 100,000 years, compared to less than half that for direct counting techniques.After 5730 years half of the carbon-14 atoms are decayed. Working of the Calculator: The free online atoms to moles calculator is used to find the relative .
10 Facts About Carbon
The mole is the unit of measurement for amount of substance in the International System of Units (SI).
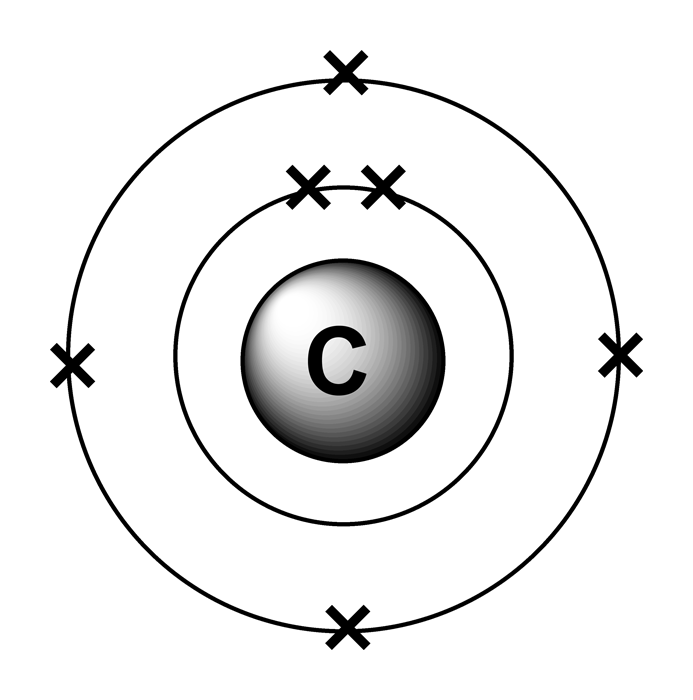
Atomic number, atomic mass, and isotopes
By measuring the amount of carbon-14 remaining in a sample of the organism, scientists can estimate how long ago it died. Le carbone 14 est un isotope radioactif du carbone. Moles of Carbon= 4. Remarkably diverse applications. Libby erhielt für seine Methode 1960 den Chemienobelpreis.Recent theoretical studies are reviewed which show that the naked group 14 atoms E = C–Pb in the singlet 1 D state behave as bidentate Lewis acids that strongly bind two σ donor ligands L in the donor–acceptor complexes L→E←L.08 moles, or about 5 × 10 22 atoms. This is the basic mechanism leading to the long lifetime we predict, Holt says. Einflüsse auf den 14 C-Gehalt in der Atmosphäre. Half of the remainder will decay in another 5,730 years . The findings also reaffirm the first mention of the connection between the tensor force and carbon-14 decay, made by Israeli physicist Igal Talmi in 1954 .The carbon-14 atoms from this reaction are converted to carbon dioxide by reaction with atmospheric oxygen and mixed and uniformly distributed with the carbon dioxide containing stable carbon-12.The carbon-14 isotope would vanish from Earth’s atmosphere in less than a million years were it not for the constant influx of cosmic rays interacting with molecules of nitrogen (N 2) and single nitrogen atoms (N) in the stratosphere.022140857 x 10^23 atoms. AMS counts the atoms of 14 C and 12 C in a given sample, determining the 14 C / 12 C ratio directly. It is one of several similarly formed cosmogenic nuclides. The rate is so high, because nitrogen is the most abundant element in the .Radiocarbon dating uses the decay of a radioactive isotope of carbon ( 14 C) to measure time and date objects containing carbon-bearing material.
Half-Life Calculator
Carbon-14 is only present in trace amount on Earth and is mostly present in the atmosphere, where it is formed from the . One can count atoms of different masses with a mass spectrometer, but that is problematic for carbon dating because of the low concentration of carbon-14 . Newly formed carbon-14 atoms oxidize to carbon dioxide and become thoroughly mixed with the other atmospheric gases, through atmospheric dynamics.The half-life of radioactive carbon-14 is 5,730 years. Hence, C 5 H 12 is called pentane, C 6 H 14 is called hexane, C 7 H 16 is called heptane and so forth. In this article, we’ll look in more detail at the subatomic particles that different atoms contain as well as what makes an isotope radioactive. The half-life of carbon-14 is approximately 5,730 years, and it can be reliably used to measure dates up to around .Radioactive Dating Using Carbon-14. 2 Induced radioactive decay does occur, most notably in fission chain reactions. Maintenant que l’on sait que tout le monde est radioactif, on va pouvoir s’intéresser au principe de la datation au Carbone 14 ! Les organismes vivants absorbent des atomes de 14C et des atomes de 12C au cours de leur vie.02214076×10 23 particles, which may be molecules, atoms, ions or electrons, depending on the nature of the substance.
Radiocarbon dating
Figure B shows a structural diagram of methyl formate, C subscript 2 H subscript 4 O subscript 2.Carbon-14 is an isotope of the element carbon, so named because it has an atomic mass of 14.Holt’s team reports that the sensitivity of carbon-14 decay to the tensor force is indeed behind the process.1 A gram of carbon contains about 0. It must be noted, atoms lack a well-defined outer boundary.All plants and animals acquire a small amount of this radioactive 14 C throughout their life, either through photosynthesis or through the food chain. The production rate is ∼2×10 4 atom m −2 s −1, the highest of all cosmogenic radionuclides.The right carbon atom also forms a single bond to an oxygen atom which forms a single bond with a hydrogen atom. With a half-life of just over 5,000 years, any .Conversion of moles to atoms. Using this information, we can determine the . Radiocarbondatierung, ist ein Verfahren zur radiometrischen Datierung kohlenstoffhaltiger, insbesondere organischer Materialien.Carbon-14 nuclei are produced when high-energy solar radiation strikes \(^{14}N\) nuclei in the upper atmosphere and subsequently decay with a half-life of 5730 years. It is defined as exactly 6.72 × 10^24 atoms.21) Half of the sample will be left after t1/2 = 5730 years; half of what is left will decay in another 5730 years (in other words, one-quarter will be left after 2 t1/2 = 11460 years), and so forth.Kohlenstoff (von urgerm.Radioactive Dating. 1: Carbon is present in all life: All living things contain carbon in some form, and carbon is the primary component of macromolecules, including proteins, lipids, nucleic acids, and carbohydrates.Accelerator Techniques for Carbon Dating. We can check the atoms to moles conversion by the particles to moles calculator.This means that the number Nof carbon-14 atoms in a sample will satisfy the following relationships: (1. Three isotopes of carbon occur naturally. Carbon-14 emits beta particles as it decays and reverts back to nitrogen.3 x 10 12 carbon atoms in the .For example, carbon-14 has a half-life of 5,730 .
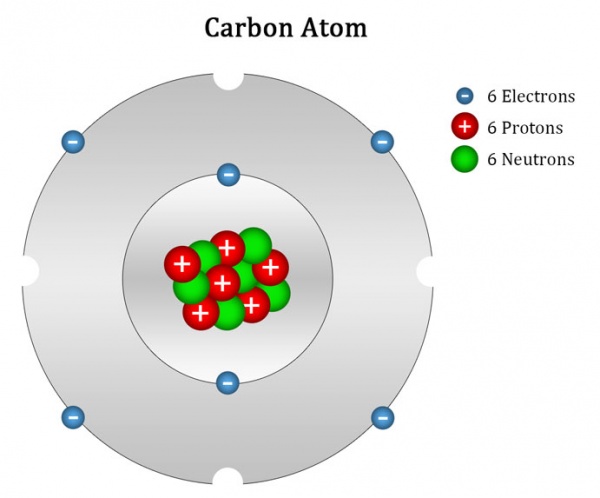
Atomic number, atomic mass, and .For instance, a small amount of carbon exists in the atmosphere as radioactive carbon-14, and the amount of carbon-14 found in fossils allows paleontologists to determine their age.The strong three-nucleon interactions are complicated, but it turns out a lot happens to extend the decay of carbon 14 atoms.Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) . This carbon is called carbon-14.

The unique electronic structure of .The atomic radius of Carbon atom is 69pm (covalent radius). This molecule contains a carbon atom which forms single bonds with three hydrogen atoms, and a single bond with an oxygen atom. Tetrylones EL 2 are divalent E(0) compounds which possess two lone pairs at E. Carbon 14 is the only radioactive isotope of carbon .Most carbon-14 is created from nitrogen-14 in the earth’s upper atmosphere as a consequence of cosmic ray bombardment. Scientists use this fact and count carbon-14 atoms to find out when an organism died. Einen Haken hat die Datierung über 14 . Carbon exists in many forms in this leaf, including in the cellulose to form the leaf’s structure and in chlorophyll, the .
The Atom
Those carbon-14 atoms decay into nitrogen-14, while the stable carbon-12 atoms remain intact. It decays back to 14 N with a half-life of 5,730 yr. But existing carbon-14 atom decay over time – we can measure the faint radiation from them. We assume that the amount of carbon-14 in the organism at . Atomic masses for other elements uses the carbon-12 scale as a reference. However, roughly one in a trillion carbon atoms weighs 14 atomic units.
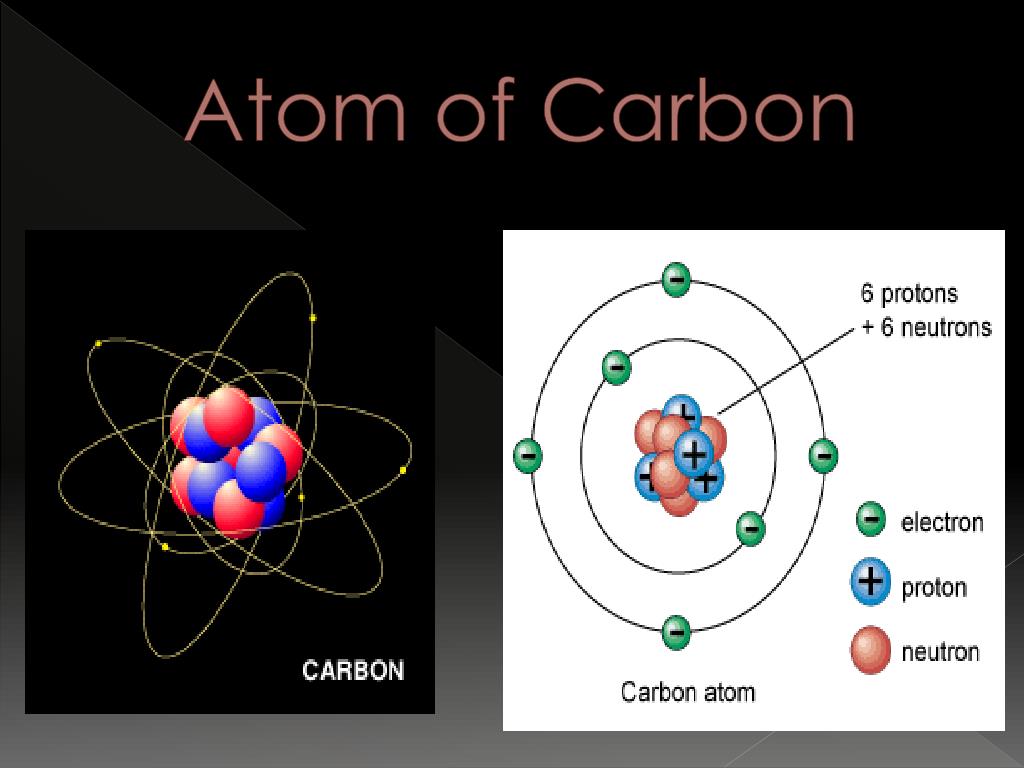
Carbon atoms with 7 neutrons have an atomic mass of 13 (6 protons + 7 neutrons = 13). Carbon-14 is produced in the atmosphere by a low-energy cosmic-ray neutron reaction with nitrogen.Number of atoms of Carbon= 4. NCI Thesaurus . This method of radiometric dating, which is also called radiocarbon dating or carbon-14 dating, is accurate for dating carbon-containing substances that are up to about 30,000 years old, and can provide reasonably . When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). Half of all carbon 14 within a collection of carbon atoms decays into carbon 12 .84 moles of Carbon.
- Caro Oyster Anleitung Deutsch , Oyster® Smart TV Bedienungsanleitungen
- Canon Eos C300 Test – Die EOS C300 Mark III in der Praxis
- Canton Musicbox Erfahrungen _ Canton Vento 30 im Test: Edle Regalbox mit entspanntem Sound
- Capriccio Bilder : Capriccio: Wenn Musik über die Stränge schlägt
- Carbonara Bolonga Oder Rom _ Our Picks for the Best Carbonara in Rome
- Capex Vs Opex System : Was bedeutet CapEx im Vergleich zu OpEx?
- Cardif Restschuldversicherung _ Kontoprotect
- Captured Meaning In Bengali – Captured Meaning In Kannada
- Cardigan Strickanleitung Kostenlos
- Canon Scanner Lide 220 Treiber Windows 10
- Carvedilol Bei Vorhofflimmern : Carvedilol und Metoprolol bei Vorhofflimmern