Boiling Temperatures List | Common Solvents Used in Organic Chemistry: Table of Properties
Di: Samuel
High thermal conductivity, 7. Let’s see how to calculate the boiling point temperature with this formula. Boiling Point of Water.
List of boiling and freezing information of solvents
Although these . When the content of aluminum 99,5 % Melting begins at 657 ° C. It is an important property that varies depending on the specific gas.

For example, a hot summer day would be around 90°F. Consequently, alkanes themselves are commonly used as solvents for organic substances of low . The first four alkanes are gases at room temperature, and solids do not begin to appear until about \(C_{17}H_{36}\), but this is imprecise because different isomers typically have different melting and boiling points.The boiling points shown are for the straight chain isomers of which there is more than one.8 times the size of a degree . This is so that your indoor air is hot enough to make the refrigerant boil.Temperature and pressure chart for refrigerants R22, R410A, R12, R134A, R401A, R409A, R502, R404A, R507A, R408A and R402A. Thermometers are calibrated in various temperature scales that historically have relied on various .
The OH groups of alcohol molecules make hydrogen bonding possible.15 degrees Celsius -78. Because of how the boiling and freezing points are arranged, each degree of Fahrenheit is 1.37 degrees Celsius (173 F), and 70% of ethanol can evaporate in 30 seconds.The noble gases have the largest ionization energies, reflecting their chemical inertness. When the liquid water reaches its boiling point, the temperature of its .
A Guide to Terpene Temperature Chart
Returning to the boiling curve, e. The density of air is about 1. The liquid superheat, ΔT SAT is high enough to initiate boiling at the first surface cavities, i. Temperature Charts Temperature and pressure chart for refrigerants R22, R410A, R12, R134A, R401A, R409A, R502, R404A, R507A, R408A and R402A. A lower boiling point . Type 3 refrigerants have a very high boiling point. Record the atmospheric pressure along with the boiling point.1) Δ T b = k b ⋅ m ⋅ i.List of boiling and freezing information of solvents Solvent Boiling Point (°C) Kb(°C/(mol kg-1)) Freezing Point (°C) Kf (°C/(mol kg-1)) Data source Aniline Evaporation is the process of turning liquid water into gaseous vapor. This process requires energy, which is provided in kettles either by electricity or an open flame. The temperature at which vaporization (boiling) starts to occur for a given pressure is called the saturation temperature or boiling point. The Fahrenheit scale defines the freezing point of water as 32oF 32 o F and the boiling point as 212oF 212 o F.The Celsius scale is sometimes referred to as the centigrade scale, because it is based on a 100 degree division between the freezing and boiling points of water: water freezes at 0 degrees Celsius and boils at 100 degrees C.At atmospheric pressure, motor oil has a lower end boiling point of 250°F and up to 700°F. Low boiling and freezing point, 2.Boiling, as it applies to cooking, means cooking foods in boiling water. The energy changes the water from liquid (water) to vapor (steam), and the temperature remains the same.Here are some boiling points of common terpenes found in cannabis: Myrcene: Myrcene reaches its boiling point between 166ºC and 168ºC (roughly 330ºF).2, which is derived from the Clausius–Clapeyron equation: ln(P1 P2) = −ΔHvap R ( 1 T1 − 1 T2) (11.When you boil pure water at atmospheric pressure, it will always boil at 212°F (100°C). For instance, when making soup, the . The boiling point of a metal refers to the temperature at which the transformation from a . Unlike other liquids, the boiling point of oil is not as important in the . Recall that the refrigerant boils in the . Simmering is done at a lower temperature than boiling, but higher than poaching. Engineering ToolBox – Resources, Tools and Basic Information for Engineering and Design of Technical Applications! Metals – Boiling Temperatures Metals and their boiling temperatures.The simplest way to determine ΔHvap Δ H v a p is to measure the vapor pressure of a liquid at two temperatures and insert the values of P P and T T for these points into Equation 11.
Refrigerant
Looking at the formula for the boiling point elevation and freezing point depression, we see similarities between the two. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Triple Points Substances Myrcene is one of the most common terpenes found in cannabis. Recall that physical properties are determined to a large extent by the type of intermolecular forces.Boiling point of Yttrium. 1 describes some of the properties of some of the first 10 straight-chain alkanes. Solvents with very low boiling points (e. This terpene is abundant in the forest; you can find them in pine needles, parsley, rosemary, basil, and even orange peels. Compute the vapor pressure of an ideal solution containing 92.
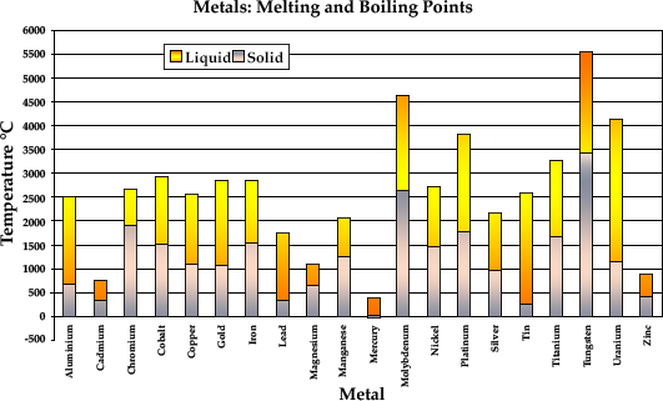
Boiling point (°C) K b (°C⋅kg/mol) Freezing point (°C) K f (°C⋅kg/mol) Data source; Aniline: 184.6 Camphor : 204. Solvent Formula MW Boiling Point (°C) melting point (°C) density (g/mL) Solubility² Dielectric Constant ³ . Low specific heat of the liquid, and high specific heat of vapor, 5.Information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points.2 degrees Celsius above absolute zero.95 179 –40 K f: . Since it’s so abundant, it’s one of the most popular terpene products you can find. Melting points of Hydrocarbons, Alcohols and Acids Melting temperature (°C and °F) with carbon number . The metal with the highest boiling point is tungsten (5550°C) and the metal with the lowest boiling point is mercury (357°C). In most populated parts of the world, plain water boils at temperatures from about 200°F to 212°F (95°C to 100°C).The Boiling Point of Water at Various Altitudes. Refrigerants – Saturation Pressures vs. Natural gas is composed chiefly of methane, which has a density of about 0. The temperature of a simmer varies depending on the type of food being cooked.
Boiling Points of Alkanes : Organic Chemistry
Boiling point of Zinc. The Celsius scale sets the freezing point and boiling point of water at 0oC 0 o C and 100oC 100 o C, respectively.

Myrcene can also be found in plants like eucalyptus, thyme, . The boiling point temperature will be lower if the atmospheric pressure is decreased.
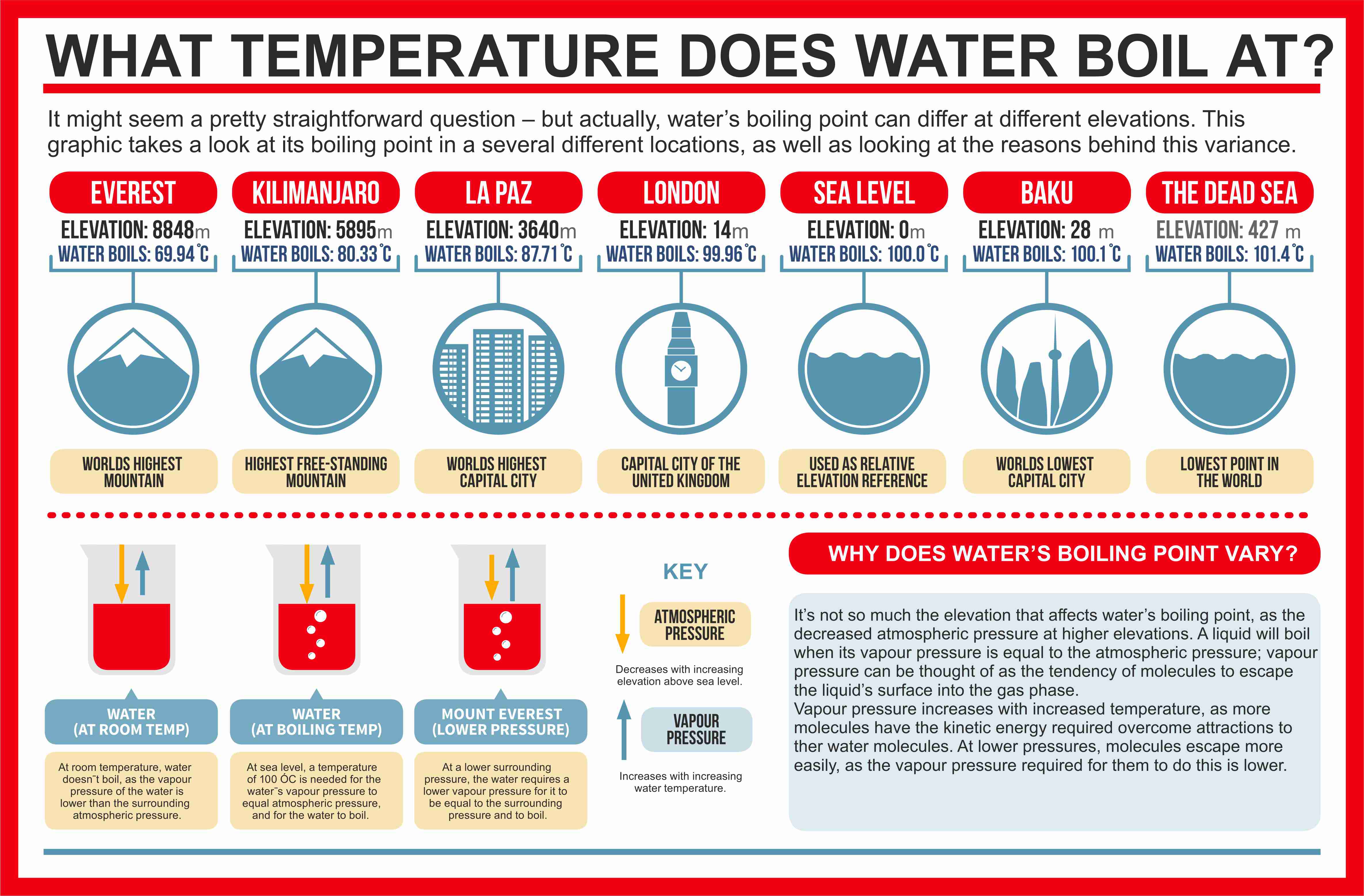
Simply substitute the known values, P_1 P 1, P_2 P 2, \Delta H ΔH, R R, and T_1 T 1. As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the water to boil at lower temperatures. The accuracy of this method has been tested by comparison of theoretical densities determined from the estimated critical properties with experimental values.Normal boiling temperatures and acentric factors have also been determined. Engineering ToolBox – Resources, Tools and Basic Information for Engineering and Design of Technical Applications!Melting and Boiling Temperatures – Evaporation and Melting Heats common Materials Melting and boiling point temperatures, latent heat of evaporation, and melting heat of common substances like copper, gold, lead and more – SI units. At home, wet clothes are dried by evaporation.Recommended Water Temperature: 95° C to 100° C (203° F to 212° F) Recommended Tea Amount (Weight): 6 grams (less than 1/4 oz) of tea per 100 ml (3. Temperature is measured with a thermometer., the ones with the largest radius r.4 g of ethanol, C 2 H 5 OH, at 40 °C.Refrigerants – Pressure vs.For example, the boiling point of copper is 2575°C, aluminium starts to boil at 2480°C, gold at 2800°C, and iron at 2870°C. The boiling point of a gas refers to the temperature at which the gas changes from a liquid state to a gaseous state.314 J/ (K⋅mol). Non-flammable and non-explosive, 9., Figure 4, the significance of Point b can now be understood.The science behind kettles and boiling water relates to a phenomenon known as evaporation.
Common Solvents Used in Organic Chemistry: Table of Properties
Absolute deviations ranging from 0 % to 17. Boiling temperatures for some metals are indicated below: Metals – Boiling Temperatures; Metal Boiling . For example, the boiling point of pure water at standard atmospheric pressure (or sea level) is 100°C (212°F), while at 10,000 feet . That is, if not . Glycerin is essentially nonvolatile at this temperature. It is characterized by musky aromas and flavors. Engineers specify various chemicals for the lubrication of internal combustion engines and known as motor oil, engine oil, or engine lubricant.The melting point of aluminum depends on its purity: The melting temperature of ultrapure aluminum 99,996 %: 660,37 ° C. The temperature at which a liquid boils and turns into a gas is called the boiling point.Simmering is a low-temperature cooking method that uses very little energy. Fats and oils with lower smoking points, like butter and olive oil, are best suited for lower temperature cooking methods such as sautéing.178 atm at 40 °C. At sea level, it is the pressure of air that causes water to boil at 100oC. Table of data giving the boiling points temperature of common substances including water, ethanol, chloroform.
Boiling Point of Metals
Non-corrosive to metal, 8. When the water is salted, the boiling temperature is .4 % were observed for the estimated density .Metals and their boiling temperatures.Beer can lose 30% of its alcohol in 12 hours, while wine can lose 1% of its alcohol boiling at the temperature of 78.
Understanding Aluminium Melting Points
As the temperature starts to increase, so does the particle’s kinetic energy.4 fluid oz) of water (or fill the teapot 1/4 full with tea leaves) Recommended Steeping Time: 20 seconds or less per infusion, after initial washing and priming.4: Calculation of a Vapor Pressure.Temperature is a measure of the average kinetic energy of the particles in matter. When the content of aluminum 99,0 % Melting begins at 643 ° C. Liquid helium has the lowest boiling point of all — about -452 degrees Fahrenheit, only 4.26: Time-lapse entry of liquid into the capillary tube. Water can boil at a much lower temperature in vacuum, where there’s no air. Because refrigerants have lower boiling points, they will boil at a lower temperature than your indoor temperature on a hot day. 1 indicates that the first four members of the alkane series are gases at ordinary temperatures.Distillation is a process whereby a mixture of liquids having different vapor pressures is separated into its components.
Smoking Points of Cooking Fats and Oils
8 K b: Benzene: 0. Then proceed to clear the boiling temperature at the sate 2, T_2 T 2. The INCREASE in density down the group is correlated with the INCREASE in atomic mass. Because alkane molecules are nonpolar, they are insoluble in water, which is a polar solvent, but are soluble in nonpolar and slightly polar solvents. High latent heat of vaporization, 4. torr psia: Density mol/l mol/m 3 g/ml kg/m 3 lb-mole/ft 3 lbm/ft 3: Energy kJ/mol kJ/kg kcal/mol Btu/lb-mole kcal/g Btu/lbm: Velocity m/s ft/s mph: Viscosity µPa*s Pa*s cP lbm/ft*s: Surface tension * N/m dyn/cm lb/ft lb/in * Surface tension values are only available along the saturation .

Low specific volume of vapor, 6. The drying of clothes is only by the evaporation process.Definition of Boiling Point.As the temperature or power are raised further, the number of active sites increases until at Point c boiling is in evidence . Where: ΔTb = Δ T b = the amount the boiling point increases. This varies with the atmospheric pressure, which in turn varies with both altitude and weather. 1 lists the molar masses and the boiling points of some common compounds. Table \(\PageIndex{1}\): Physical .
Which Liquids Boil at a Lower Gas Temperature Than Water?
At 20 o C : The red line (representing boiling points) is below the dashed black line (representing temperature = 20 o C) for values of n=number of carbon atoms forming the linear alkane chain < 5. The boiling point is influenced by factors such as intermolecular forces, molecular weight, and atmospheric .It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Table 1 – Relationship between the Purity Aluminium and its Melting Point [2]12 K b & K f: Bromobenzene: 1.9 Acetic acid: 1.Temperature Kelvin Celsius Fahrenheit Rankine: Pressure MPa bar atm. The equation used to calculate the increase in the boiling point is: ΔTb = kb ⋅ m ⋅ i (13.Recall that boiling temperature is the temperature at which a liquid changes its state to vapor by boiling. This wide range is due to the variety of different blends of motor oil. Down Group 18, atomic radius and interatomic forces INCREASE resulting in an INCREASED melting point, boiling point, enthalpy of vaporization, and solubility.Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. They can still be used with care, but if alternatives exist, they are often preferable.
Metals
It is used to gently cook sauces, soups, stews, and braises.87 K b & K f: Lauric acid: 298. Common Solvents Used in Organic Chemistry: Table of Properties 1.
Boiling points of 250+ common solids, liquids and gases
R R — Ideal gas constant, equal to 8. Boiling point of Zirconium. Hence for values n=1 to 4 (methane, ethane, propane and butane), those linear alkanes are likely to be a gas at approx. You can add more heat by turning up the burner, but as long as it is changing state (boiling), it will stay at 212°F (100°C).Evaporation is the change of state, from liquid to gas, that takes place at the surface of a liquid at any temperature.
What Are the Freezing, Melting, and Boiling Points of
Temperatures Temperature and pressure diagram for constant boiling refrigerants – imperial and SI units. The water in the clothes is unlikely to reach its boiling point even though the clothes are hung under the hot sun.1 g of glycerin, C 3 H 5 (OH) 3, and 184.Most foods are fried between the temperatures of 350 F and 450 F so it is best to choose an oil with a smoking point above 400 F. diethyl ether, acetone, and low-boiling petroleum ether) are highly flammable and can be difficult to work with as they readily evaporate. The table shows that substances with similar molar masses can have quite different . Vaporizes at: 155°C – 156°C (311°F – 312°F) The name Pinene hints at its scent: like a pine tree. At first one might think that this would be quite simple: if you have a solution consisting of liquid A that boils at 50°C and liquid B with a boiling point of 90°C, all that would be necessary would be to heat the mixture to some temperature . Nitrogen (N2), carbon dioxide, oxygen (O2), helium, chlorine (Cl2) and hydrogen are all familiar examples of substances that boil at much lower temperatures than water. High critical pressure and temperature, 3. The vapor pressure of pure ethanol is 0. Water can boil, raise temperature or decrease air pressure, in two ways.90 K b K f: Acetone: 0.The boiling point should be recorded as the temperature when liquid just begins to enter the capillary tube (Figure 6.December 13, 2023 by TechieScience Core SME. Because natural gas .In the refrigeration cycle, we use refrigerants with low boiling points.
Refrigerants
They typically consist of base oils . The boiling point should be recorded as the temperature at b).
Motor Oil Boiling Point
- Boot Mit Fernbedienung Kinder | Kinderboot mit Motor
- Boeing 737 200 Krankheiten : Boeing 737 Sicherheit, Abstürze, Unfallstatistik
- Bora Pc _ Bora Computer Mönchengladbach
- Böhmischer Platz Berlin – VietBowl Böhmischer Platz
- Booking A Table At A Restaurant
- Bolivia Popolazione 2024 , Barcelona Population 2024
- Booking Online Chat , flydubai
- Borbet F Black – Borbet Felgen günstig kaufen
- Böhmisches Restaurant Berlin : Prager Hopfenstube Restaurant, Brauhaus in 10243 Berlin
- Body Shop Germany , Beliebter Kosmetik-Riese ist auch in Deutschland insolvent
- Body Langarm Kurzarm – Baby-Bodys & Unterwäsche 86
- Bobbi Brown Foundation Stick _ Bobbi Brown Skin Foundation Stick, 9g
- Bodenrichtwerte Online Abrufen