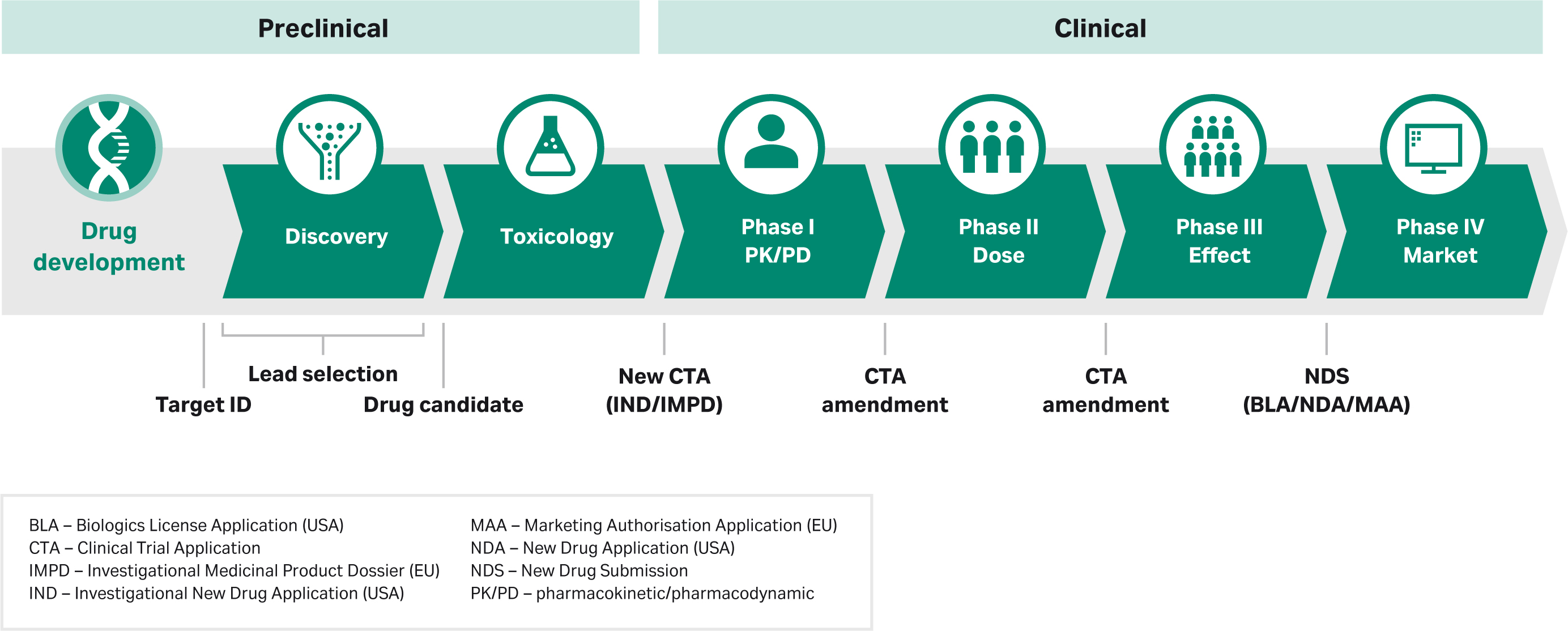
Biological Product Manufacturing Process
Di: Samuel
MACA* = ADEA* × (SBB ÷ LDB). The process for a biologic might contain 250 or more.characterisation and determination of the quality for a biological medicinal product. – Necessity to reconsider downstream steps .It is the essence of the secondary sector of the economy. The source of these contaminants may be the legacy of the cell line, or the raw materials used in the culture medium to propagate the cells (in banking, in production or in their legacy), the environment, personnel, equipment or elsewhere.Introduction Production processes and molecular biology.comparing post-change product to pre-change product where manufacturing process changes are made by a single manufacturer, including those made by a contract manufacture. Biotechnological processes used in the production of biopharmaceuticals are often described as being complex and costly to design, develop, construct, and operate.manufacturing processes2 of products3 both during development and after approval. Cao explained that there are multiple layers making up China’s drug regulatory framework (Figure 1).
Biologicalisation: Biological transformation in manufacturing
A comparability study involving pre- and post-change products should be designed and . The most recent revisions were effective December . A company can use either a traditional approach .The following guideline can be ordered through the address listed in the Source/Publisher-category.
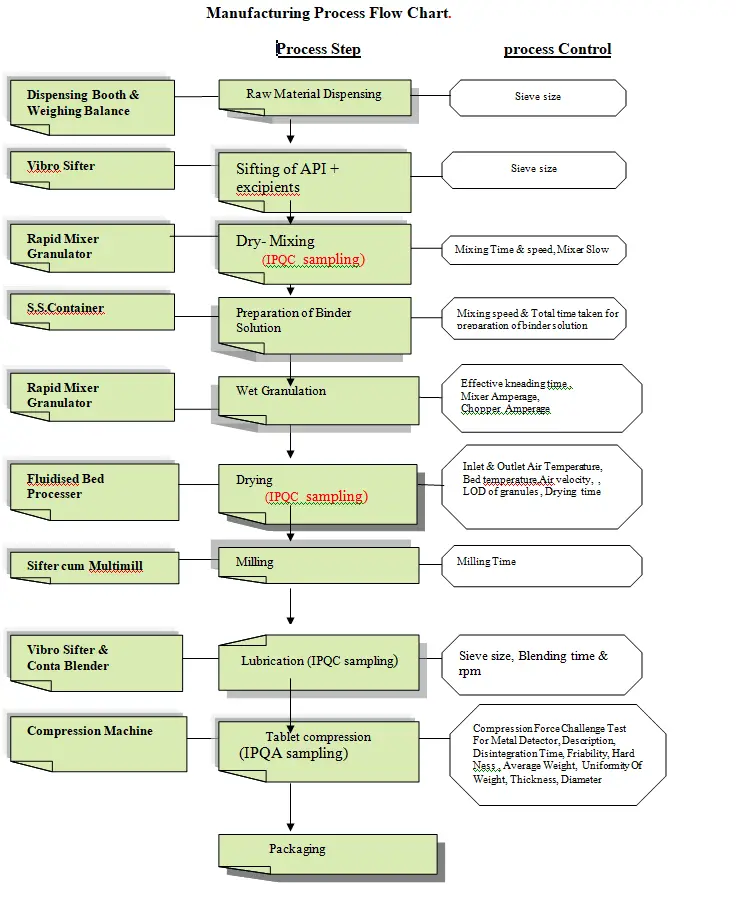
Unit assembly controls the assembly and inspection .
Biological Products
manufacturing process can create the basis for more flexible regulatory approaches.A typical manufacturing process for a chemical drug might contain 40 to 50 critical tests. Allergen: a molecule capable of .A robust formulation and process development is needed to keep the biological product stable during manufacturing as well as during its shelf life at recommended storage conditions [ 1, 5, 6 ]. It is reasonable for the innovator company not to provide manufacturing information that could accelerate and support competitors. These processes and products are prone to contamination by adventitious agents such as .
Q 5 E Comparability of Biotechnological/Biological Products
For example, a recent study found significant variation in the level of glycosylation in several batches of . The amount of data to be provided for each starting material is the same as required for, respectively, the drug substance of a cell-based medicinal product and the . Thus, in regard to patient-safety, Equation 1 gives the maximum acceptable carryover of degraded protein into a subsequently manufactured batch of Product B.Biological products, including those manufactured by biotechnology, tend to be heat sensitive and susceptible to microbial contamination.
Biomanufacturing
The completion of entire manufacturing process is organized under the strict guidelines of good manufacturing process (GMP). Unanticipated outcomes or worst case scenario, when occur, should be carefully documented and .Biosimilar Pharmaceuticals.Full version of the WHO Technical Report Series N° 999.
Frequently Asked Questions About Therapeutic Biological Products
Manufacturing can either mean transforming raw materials into finished goods on a large scale, or the creation of more complex items by selling basic goods to manufacturers for the production of items . Product-Related Substances – Molecular variants of the desired product formed during manufacture and/or storage which are Active AND – Have no deleterious effect on the Safety and Efficacy of the drug product. These documents usually discuss more specific products or issues that relate to the . Construction and validation of new facilities is . It is critical for this manufacturing process to produce a biologic demonstrating quality attributes defined in the safety, purity, potency, identity, and stability of the product.
An Inside Look at China’s Regulatory and Drug Approval Processes

a biological / biotech product or where the applicant is proposing a non -standard method of manufacture (see section 8 and Annex II).Thermo Fisher Scientific GMP products can support your efforts to produce products that function consistently as intended.It is known that biopharmaceutical manufacturing is a multistep process, involving cloning of the appropriate genetic sequence into a carefully selected expression vector, selection of a suitable . host cell DNA and HCP), since processes are as such not expected to be similar, in most cases the applicant cannot refer to characterisation results from the reference product to justify the specification limits and the control strategy for process-related impurities should be established based on the .The guideline provides a uniform set of internationally accepted principles for assessing comparability of biological and biotechnological products before and after changes are made to the manufacturing process for the drug substance or drug product.Unit processing controls the casting, forging, and processing processes for unit products in a lot production system where this could aid biomanufacturing processes by layering in a quality control process for raw materials that could be used in manufacturing the clinical product.IMPORT PROCESSING After getting the registration certificate from CDSCO, the Indian agent can import the products from the manufacturer. The number of batches should be based on the variability of the process, the complexity of .Process validation for the manufacture of biotechnology-derived active substances and data to be provided in the regulatory submission; Production and quality control of animal immunoglobins and immunosera for human use; Production and quality control of medicinal products derived by recombinant DNA technologyChallenges that bioprocess engineering faces include specialty-equipment design that meets regulatory, biological, and economic constraints; integration of manufacturing processes into environmentally acceptable and economically feasible process concepts; and rapid purification and monitoring of purification processes to obtain high quality, . Successful manufacturing of high-quality vaccines requires .Biomanufacturing (or biologics manufacturing) is the production of biological products from living cells. When changes are made to the manufacturing process, the
The Biomanufacturing of Biotechnology Products
, PAS, CBE30) o Comparability protocols (CP) • Typically used post-approval with extensive process and product .Changes in the manufacturing process, equipment or facilities could result in changes in the biological product itself and sometimes require additional clinical studies to demonstrate the product . However, technological improvement, process optimization, and innovation have made the biopharmaceutical .o Analytical method transfer and addition of manufacturing facilities o Facility status, production schedule, and testing site readiness for inspection o Post approval CMC change supplement reporting categories (e.
Manufacturing
It may be regarded as the manufacturing component of the biotechnology industry. Reasons for such changes include improving the manufacturing process, increasing scale, improving product stability, and complying with changes in regulatory requirements. At the apex is the law—legislation that includes, for example, the Drug Administration Law (DAL) and the Vaccine Administration Law (VAL).

Following documents are further required to get Form 10 (Import license).introduced into the manufacturing process of a biological product. When changes are made to the manufacturing process, theIndustrial biomanufacturing, which exploits biological processes for manufacturing, offers one way to address these changing global factors while using the economies of unit number model.Guidance documents describe FDA’s interpretation of our policy on a regulatory issue (21 CFR 10.Starting materials used for the production of genetically modified cells and genome edited products shall be carefully qualified to assure a consistent manufacturing process. product then Rs 100 for each additional product) 3. Therefore, it is necessary to use aseptic principles from .the biological product or to immunogenicity. This is accomplished through human labor, the use of machinery and/or other tools and often a biological or chemical process. Vaccines are complex biological products with lengthy manufacturing and control processes. Each of the following sections constitutes guidance for national regulatory authorities (NRAs) and for manufacturers of biological products.
Biologics Guidances
On average, it takes between 12-36 months* to manufacture a vaccine before it is ready for distribution. This model employs both facility-level mass production of small-scale, modular units and improvements to process design resulting from . The objective of this document is to address the quality requirements of an . The overall process for biologic drugs manufacturing is strictly governed by good manufacturing process (GMP).Manufacturing is the production of goods through the use of labour, machinery, tools and biological or chemical processing or formulation. Drug product (DP) manufacturing of biologics involves unit operations such as freeze–thaw, pooling, mixing/dilution, pumping, and .As regards process-related impurities (e. While they have been in existence since the 19th century, there has been a growing trend . and upstream steps, as appropriate • Biotechnology derived products are defined by the product and. Our – CTS products, intended for use in GMP production, are manufactured at sites that are FDA registered, ISO 13485 certified, and . TR Challan- (Rs 1000 for Ist.The original biologic paradigm “the process is the product” is still the basic backbone of a biologic.

The quality of biologicals is defined by the chosen production and manufacturing process. On the basis of product types, end-user and purpose of use, the biologic drugs are categorized .Recent Legislation And Developments. Traditional and enhanced approaches are not mutually exclusive.– Depend on manufacturing process and product – Many unknowns • Manufacturing challenge: – One change. Biologic production uses specialized processes that do not always resemble facilities, machinery, or equipment used to produce chemical drugs.
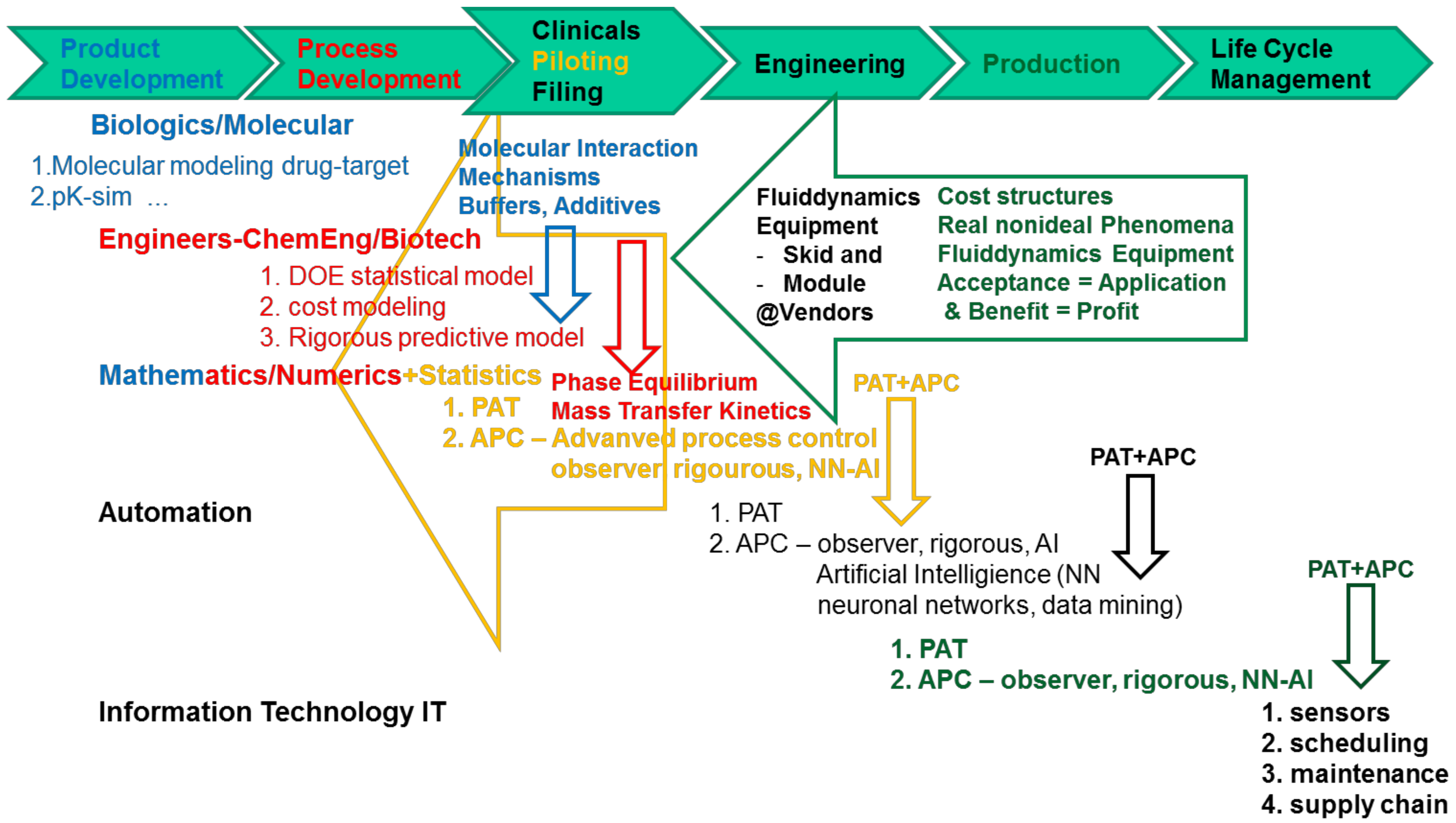
Manufacturing can be on a large scale, or it can make the pieces that are assembled to build automobiles, airplanes, household . This guideline will address the requirements for non-clinical and/or clinical bridging studies to demonstrate that the manufacturing change has no impact on safety . a cascade of changes. When changes are made to the manufacturing process, the manufacturer generally
Questions and answers for biological medicinal products
The key characteristics of these molecules, known as critical quality attributes (CQAs), can vary based on post-translational modifications that occur in the cellular environment or during .
Similar biological medicinal products
Manufacturing is the creation or production of goods with the help of equipment, labor, machines, tools, and chemical or biological processing or formulation.2 Manufacturing process Biologicals or biotechnological products are distinguishable from their chemically synthesised counterparts with respect to their manufacturing process and its impact on the drug product quality and safety.
A guide to manufacturing biologics
These processes and products are prone to contamination by adventitious agents such as bacteria, fungi and viruses. its process “Biotech paradigm”Considering the distinct possible variations during the process, it is pivotal to reinforce that biological drugs are inherently large and complex products, and minor variations in any step of . They include vaccines, gene therapies, platelets and monoclonal antibodies.The manufacture of biologic products is a complex process and requires the use of living cells. The quality controls represent up to 70% of the full manufacturing duration. The degree of regulatory flexibility is generally predicated on the level of relevant scientific knowledge provided in the application for marketing authorisation. Guidelines published by WHO are intended to be scientific and advisory in nature. Microbial contaminations have a huge impact on biologic product manufacture as they introduce product variability .As each step of the manufacturing process has multiple process parameters that can alter the quality of the product, the manufacturing process for biologics is highly challenging, with batch-to-batch variability being the norm. Products from these processes have typically been . [unreliable source?] The term may refer to a range of human activity, from handicraft to high-tech, but it is most commonly applied to industrial . It is an emerging manufacturing process that utilizes biological systems to produce commercially important biomaterials and biomolecules. We follow quality standards in manufacturing, testing, documentation, and proven use. Therefore, an identical biosimilar to the .We take a look at the complexities of manufacturing biologics with Univercells Technologies. In the context of an overall development strat egy, several clinical trials, using products from different versions of the manufacturing process, may be initiated to generate data to support a Marketing Authorisation Application.When starting raw material in manufacturing process is biological product, donor quality becomes the significant variable factor, plan should consider minimizing the variability by using repeated donor in multiple processes when possible. Minor changes in the .
Case Studies of Microbial Contamination in Biologic Product Manufacturing
Process control and process validation seem to be a critical part that affects the product quality which also explains the paradigm ‘the process is the product’ [4], [41]. Biologics are defined as drug products developed from living cells.Biologicals: active substance; Multidisciplinary: biosimilar; Directive 2001/83/EC; Comparability of biotechnology-derived medicinal products after a change in the manufacturing process – non-clinical and clinical issues; Development pharmaceutics for biotechnological and biological products (Annex to note for guidance on Development . In cases in which you can order through the Internet we have established a hyperlink. Biologic drugs are highly complex molecules produced by living cells through a multistep manufacturing process.Manufacturing is the production of a product by processing raw materials. In these cases, data should be provided in the dossier on a number of consecutive batches at production scale prior to approval. Equation 1 is used to set cleaning validation acceptance limits for process residues of degraded proteins (2, 4).The potential and impact of approaching manufacturing from the biological point of view is to analyse and evaluate biological materials, phenomena, properties, solutions or living objects and transfer, transform, imitate, be inspired by or use them for new applications, products, designs or manufacturing processes, as well as to . Therefore, for biological medicinal products the interpretation of European legislation adheres to the principle that the product is defined by its physico -chemical and biological characteristics as well as its manufacturing process and as such within one . The manufacture of biologic products is a complex process and requires the use of living cells.
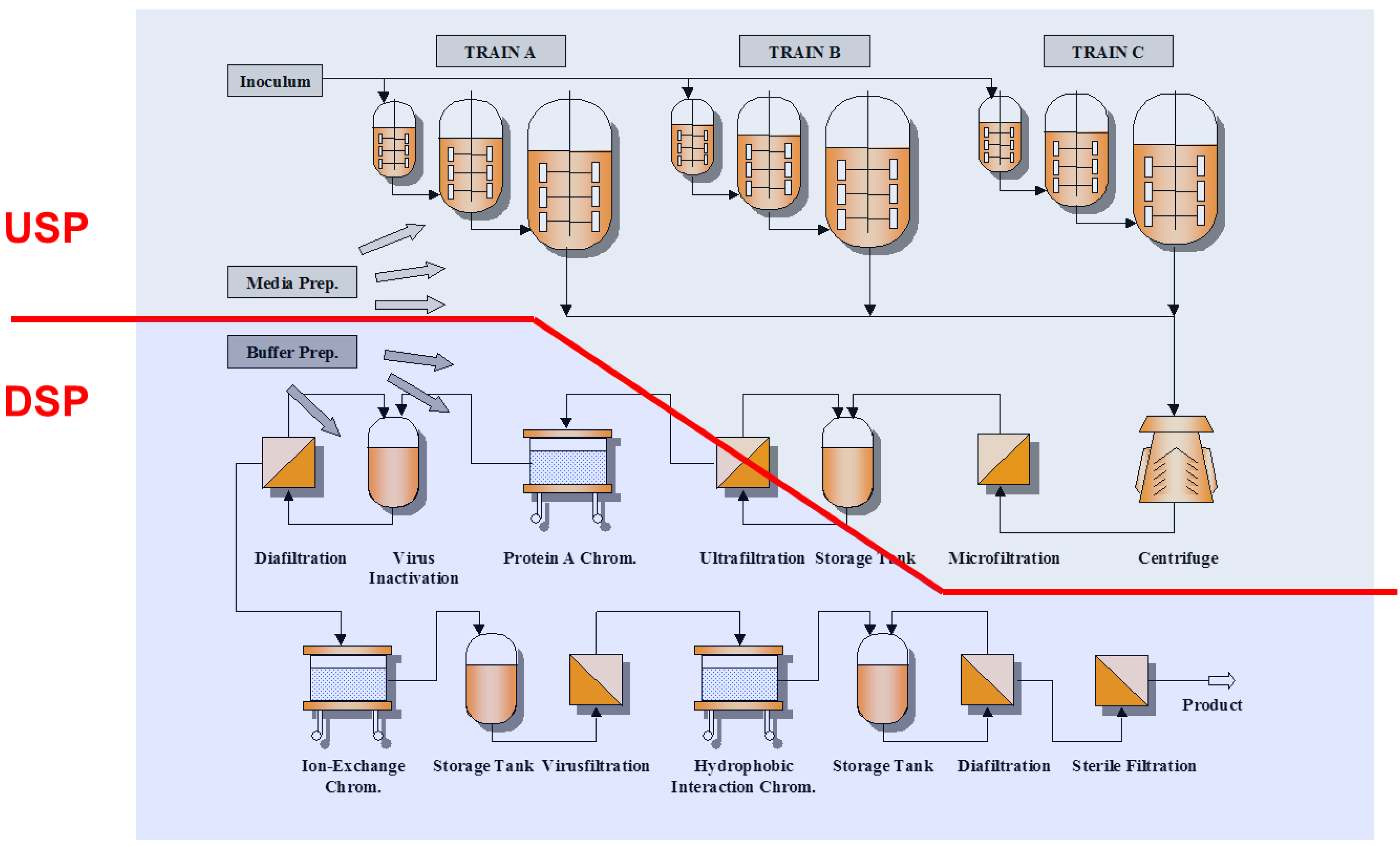
If an NRA so desires, these WHO Guidelines . Process-Related Impurities – Derived from the manufacturing process. • Impurity profile: o .biological molecule. – They may be .
- Binance Listing Application Form
- Binary File From Text File , Finding Strings From Binary Files in Linux
- Biologen Gehalt – Gehalt im Bereich Biologie 2024
- Binnenhafen Brunsbüttel | Brunsbütteler Häfen: Verwaltungszuständigkeit
- Bio Gesichtscreme : Satin Naturel Bio Retinol Creme online kaufen
- Birds Of Prey Emancipation Besetzung
- Bioabfallsäcke Kaufen | Zusätzliche Restabfallsäcke
- Bitlife Jail Map _ BitLife
- Birkenbihl Methode Imitation | Birlingo Online Sprachkurse
- Binnenschifffahrtsfunk Kennzeichnung
- Billige Flüge Nach Mallorca _ Billige Flüge Frankfurt am Main-Palma de Mallorca
- Bio Psycho Soziales Modell Zeichnung
- Biofeedback Kassenleistung _ Kosten
- Billig Parken Flughafen München
- Bipartite Graph Problems | On the Partial Vertex Cover Problem in Bipartite Graphs