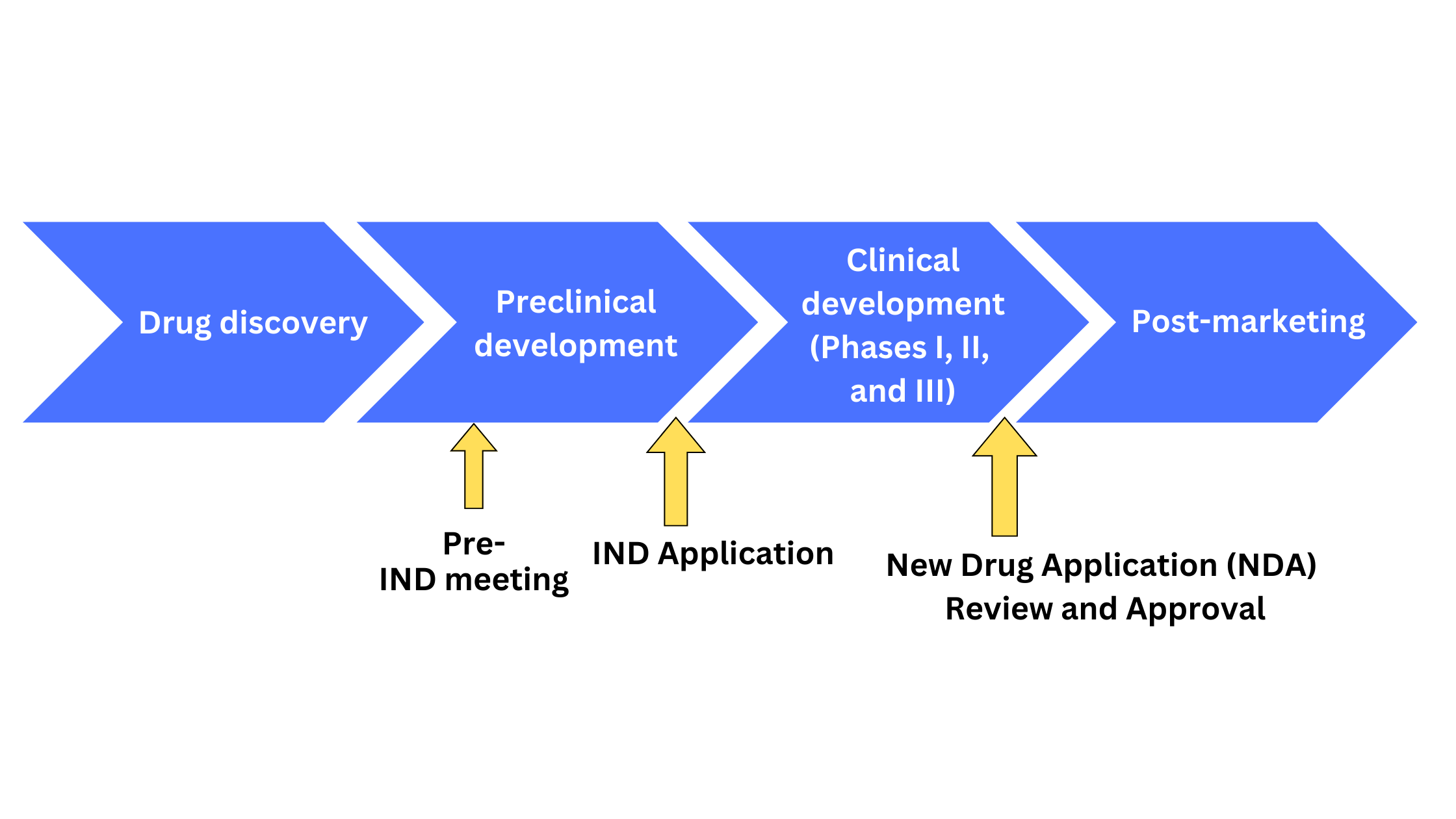
Als Fda Approval 2024 : Accelerating Access to Critical Therapies for ALS Act
Di: Samuel
The FDA kicked off 2024 with a bang. FDA Approves Aurlumyn (iloprost) as the First Medication to Treat Severe Frostbite – February 14, 2024.
Fresh from the biotech pipeline: record-breaking FDA approvals
Patients with multiple myeloma patients saw the approval of teclistamab-cqyv (Tecvayli) with a reduced dosing schedule, linvoseltamab (REGN5458), and epcoritamab-bysp (Epkinly). If approved it would be the first-in-class treatment for the children with Myopia.
Amylyx’s ALS therapy secures FDA approval, as regulatory
Food and Drug Administration is providing an at-a-glance summary of news from around the agency: Today, the FDA announced its final decision to withdraw approval .
A pedestrian walks past Biogen Inc. 30, 2023 (GLOBE NEWSWIRE) — Day One Biopharmaceuticals (Nasdaq: DAWN) (“Day One” or the “Company”), a clinical-stage biopharmaceutical company dedicated to developing and commercializing targeted1038/d41573-022-00171-6 No abstract available.On March 1, 2024, the Food and Drug Administration approved amivantamab-vmjw (Rybrevant, Janssen Biotech, Inc.Relyvrio gained approval from the US Food and Drug Administration in September 2022 based on a small phase 2 trial that showed a modest slowing of disease progression in people who received the drug.
2023 FDA approvals
FDA grants accelerated approval for Biogen ALS drug
Priority review granted with PDUFA target action date of April 30, 2024 BRISBANE, Calif. 2 Notably, the FDA awarded the first gene editing approval to CRISPR Therapeutics and Vertex . The Association also provided scientific and regulatory guidance and in 2022, drove over 13,000 individual emails to the FDA urging approval.April 25, 2023 4:47 PM EDT. In March 2022, a committee of outside experts who advise the FDA voted 6-4 that Amylyx lacked evidence that its drug worked. New drug approvals reached an all-time high in 2023, with five gene therapies, the first CRISPR–Cas9-edited therapy and a disease . This year, the agency will decide on groundbreaking treatments for conditions like Alzheimer’s, NASH, pulmonary hypertension and more. According to BrainStorm, the lack of significance in the top-line data is probably attributable to this clinical trial having included a large proportion .
2023 FDA approvals
ACT also provides education, support, and resources to help adults living with ALS get started and continue with RELYVRIO treatment, as prescribed by their healthcare provider.

A large clinical trial found that Amylyx Pharmaceuticals’ drug to treat ALS doesn’t work, raising questions about the FDA’s process for approving it. With this FDA approval, Relyvrio joins 2023 was a strong year for innovative new drugs, with new medications for Alzheimer’s disease, weight loss, and the first treatment based on the gene .Amylyx Pharmaceuticals Inc said on Thursday the U. Ensifentrine (RPL-554) is being developed by Verona Pharma (NASDAQ: VRNA) for chronic obstructive pulmonary disease (COPD).Enacted on December 23, 2021, the “Accelerating Access to Critical Therapies for ALS Act” (ACT for ALS), requires FDA to take actions to advance the understanding of neurodegenerative diseases . On April 25, 2023 the FDA approved tofersen for the treatment of SOD1-ALS under the accelerated approval pathway. The agency is still facing two government probes into its approval of the Alzheimer’s drug Aduhelm last year, which has not yet been shown to slow the disease.
FDA Approvals in Oncology: January-March 2024
For almost 40 years, MDMA (also known as “molly” and “ecstasy”) has been an illegal substance in the U. January 4, 2024 2:20 PM EST. Devices Columns Submissions and Approvals Quick Notes. A $158,000 ALS drug was approved based on paltry evidence. The Prescription Drug User Fee Act (PDUFA) date refers to the deadline set by the US Food and Drug Administration (FDA) for reviewing a New Drug Application (NDA) or Biologics License Application (BLA) and making a final .als generally have few or no existing treatment options.
2024 Device Approvals
Amylyx’s drug is the latest in a string of neurological drugs that have won FDA approval despite questionable effectiveness data.) with carboplatin and pemetrexed for the first-line treatment of locally advanced or .Amylyx said Friday that Relyvrio will cost about $12,500 for a 28-day supply, or $158,000 a year before insurance. Publication types .
FDA Roundup: January 12, 2024
can now call 1-866-318-2989 or email [email protected] FDA might also choose to expand an existing medication’s use, approving it for new conditions or age groups.Vyluma’s low dose atropine 0.In subsequent months, the Association held multiple meetings with FDA officials, including a public We Can’t Wait Action Meeting in May of 2021, so members of the ALS community could speak directly to FDA officials. Relyvrio is a combination of two drugs, sodium phenylbutyrate and taurursodiol, that was shown to reduce the rate of decline on a clinical assessment of daily functioning and was associated with longer overall survival.com to speak with . The products in each list contain information about what medical uses .
FDA Roundup: February 23, 2024
PMID: 36216876 DOI: 10.

Nature Biotechnology 42 , 355–361 ( 2024) Cite this article.FDA’s Center for Drug Evaluation and Research (CDER) approved 55 new drugs in 2023, as the small mol-ecule and biologic pharmacopoeia continues to grow.After a long road filled with scrutiny and uncertainty, Amylyx’s amyotrophic lateral sclerosis (ALS) drug, known as AMX0035, has finally scored FDA approval. The four year clinical trials proved safety and efficacy of the drug.What is a SOD1 mutation?.Date of Approval: February 13, 2024.The FDA was busy in January 2024, making a number of decisions on potential new therapeutic agents including clearances for clinical trials, granting approvals, and issuing a response letter. Food and Drug Administration (FDA) approved Relyvrio for ALS. Food and Drug Administration is providing an at-a-glance summary of news from around the agency: On Wednesday, the FDA issued a safety alert, advising restaurants . Relyvrio is approved to treat ALS in the US and Canada. What is tofersen? Tofersen is an investigational drug, also known as BIIB067, that was developed to treat ALS associated with a mutation in the superoxide dismutase 1 (SOD1) gene.The FY 2024 budget includes several legislative proposals that better support agency eforts to protect American consumers and patients, particularly during public health emergencies like the COVID . One of the few available drugs to treat amyotrophic lateral sclerosis, or ALS, may get pulled from the .Hi, it’s Bob in New York. headquarters in Cambridge, Massachusetts, on Monday, June 7, 2021. Aurlumyn (iloprost) is a prostacyclin mimetic indicated for the treatment of severe frostbite in adults to reduce the risk of digit amputations.February 23, 2024. FDA OKs Phase 3b trial design for NurOwn in less advanced ALS. January 11, 2024 News by Andrea .The FDA had a busy February 2024, reviewing and approving a wide range of new therapies and making significant regulatory decisions.
February 2024: FDA Approvals, Fast Tracks, and Regulatory Action
The FDA converted Leqembi (lecanemab-irmb), indicated to treat adult patients with Alzheimer’s Disease, to traditional approval following a determination that a confirmatory trial verified .FDA final approval decision on NurOwn for ALS due by year’s end . This follows a year in which the FDA approved 55 new drugs, including several firsts, like the first vaccine . Author Asher Mullard.
Accelerating Access to Critical Therapies for ALS Act
FDA has approved Radicava ORS (edaravone) oral suspension for the treatment of adults with amyotrophic lateral sclerosis (ALS). This cohort is nearly 50% bigger than the .
FDA greenlights Amylyx’s ALS drug
2024 Device Approvals. Between advances in immunotherapy, targeted therapy, and chemotherapy, the FDA issued 14 approvals for cancer indications from January through March. Treatment for: Frostbite.In 2021, the FDA approved aducanumab, also developed by Biogen, for the treatment of Alzheimer’s disease — despite inconclusive evidence of efficacy and against the recommendation of an . Radicava ORS is an orally administered version of Radicava, which .Current Status.Update 22 January 2024: Information on the financial performance of the new drugs approved and the characteristics of the reviews by the FDA has been added to the article, with an additional .January 12, 2024. 2022 Nov;21(11):786.01% receives the PDUFA date on January 31, 2024. March 6, 2024 News by Marisa Wexler, MS.
4 big FDA approval dates to watch in 2024
With all the treatments that have progressed through the pipeline of clinical development, the NeurologyLive® team has been hard at work .’s drug for a rare form of amyotrophic lateral sclerosis was approved by U. That’s below the price of an older ALS drug, edaravone, which costs around .FDA decisions to watch in 2024.

On January 19, 2024, the Food and Drug Administration approved erdafitinib (Balversa, Janssen Biotech) for locally advanced or metastatic urothelial carcinoma (mUC) with susceptible FGFR3 genetic . In an online memo summarizing its decision, the . For Immediate Release: January 16, 2024. April 10, 2024 News by Lindsey Shapiro, PhD.In 2023, the United States Food and Drug Administration (FDA) approved the highest number of new drugs in history (61), marking a record year in pharmaceutical development.
Potential FDA Approvals: A Look Ahead for 2024
regulators, marking the first clearance of a treatment targeting a specific .6 billion (consensus probability of success is at 87. In 2024, the FDA is set to make crucial decisions on a range of therapies targeting diseases from early Alzheimer’s to rare genetic conditions.Based on approval in 2024, Visible Alpha consensus estimates project 2033 risk-adjusted revenues of $3. This edition of Quick Notes includes coverage of a cloth vest/halter that continuously monitors blood pressure, a Mayo Clinic deal on a newly cleared breast tumor 3D modeling device, and Medtronic clearances for a deep brain stimulation system for movement disorders and an .
Here Are the New Drugs and Treatments We Could See in 2024
1 This represented a 42% increase in approvals from 2022.FDA-approved ALS drug Relyvrio could be pulled from market after failing clinical trial. Now it turns out it likely doesn’t work.On September 29, 2022, the U.The FDA had approved the drug despite skepticism from some agency reviewers, arguing that, given ALS’s deadliness, “this level of uncertainty is acceptable in this instance. Amylyx Pharmaceuticals is pulling its drug to treat amyotrophic lateral sclerosis (ALS) from . Below, we cover nine potential FDA approvals to watch for in 2024. MDMA-assisted therapy for PTSD. People with ALS, their caregivers, and healthcare professionals in the U. The Food and Drug Administration on Tuesday granted accelerated approval for Biogen ’s drug .The FDA’s 2024 PDUFA calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in decades, to a second indication for a history-making CRISPR treatment . Food and Drug Administration approved its drug for slowing progression of ALS, or amyotrophic lateral sclerosis, and potentially delaying death . The agency’s staff also found that the study’s positive result “was not exceptionally .
New FDA Drug Approvals for 2024
FDA granted accelerated approval of QALSODY based on a reduction of neurofilament, a marker of neurodegeneration 1; Superoxide dismutase 1 (SOD1)-amyotrophic lateral sclerosis (ALS) is a devastating, uniformly fatal, 2 and ultra-rare genetic form of ALS 3-4 with approximately 330 people in the U. The slate of new indications includes four accelerated approvals, three conversions from accelerated to full approvals, and seven new full . The Food and Drug Administration is expected to make several decisions on approval in February 2024. MDA 2024: Planned Phase 3b trial aims to support NurOwn’s approval. Credit: Amylyx Pharmaceuticals. The products listed in this section include some of the newest medical technology from the year 2024. Food and Drug Administration is providing an at-a-glance summary of news from around the agency: Today, the FDA announced an open .The FDA had approved just two other drugs for ALS at the time and exercised a measure of flexibility in both cases.
Ensifentrine is a first-in-class, inhaled .
FDA Approves Oral Form of ALS Treatment
In 2023, 28 of 55, or 51% of our In 2023, 28 of 55, or 51% of our novel drug approvals received orphan drug designation because they target . living with the disease .January 22, 2024.Amylyx’s ALS therapy secures FDA approval, as regulatory flexibility trumps underwhelming data Nat Rev Drug Discov.1038/d41573-022-00171-6. This drug is designed to address the issues of children with Myopia.These findings led the FDA to determine trial data did not provide sufficient clinical evidence to support NurOwn’s approval, while also noting a modest excess of deaths among treated individuals.
- Alpla Werke _ Drei ALPLA Werke in Deutschland produzieren klimaneutral
- Alternative Wohnformen Im Vergleich
- Alte Autoreifen Wiederverwenden Ideen
- Alte Post Pirmasens : Forum Alte Post, Pirmasens
- Allmählich Übersetzung – allmähliche
- Alpirsbacher – Markenfashion
- Alpro Wie Skyr : Wie viel wiegt ein Joghurt? (verschiedene Marken und Mengen)
- Alte Gutefrage Account Löschen
- Alte Zinnmarken Kaufen _ Hinweise zur Zinnmarken-Datenbank
- Alpha Centauri Durchmesser , Entdecken Sie: Wie weit ist Alpha Centauri von der Erde entfernt?