Acr Response Therapie _ Assessment and Management of Rheumatoid Arthritis
Di: Samuel
Forest plots visualizing ORs ± 95% CI for achieving ACR 20/50/70 responses in rheumatoid arthritis phase 2 studies compared to phase 3 studies.CAR-T cell therapy leads to long-term remission in lupus while maintaining vaccine response. Often clinically based, these thresholds initially seemed like a wonderful way of communicating the effect of a new treatment, that a certain number of patients would experience improvement when . Objectives To evaluate which American College of Rheumatology (ACR) response definition (ACR20, 50 or 70) should primarily be used for efficacy claims in future drug . However, only numerical differences in efficacy outcomes were seen between JAK combination therapies.
Was bringt eine Eigenbluttherapie wirklich?
The results support the selection of more stringent thresholds if later time points shall be evaluated, given their comparable discriminant but higher clinical face validity.Als Nierenparameter sollten Serumkreatinin, Urinstatus und -sediment untersucht sowie antinukleäre Antikörper (ANA) mit Hilfe des indirekten Immunfluoreszenztests (HEp-2 Zellen) bestimmt werden . The resources on this page provide information on preparing for a radiation . Um dieses sogenannte „window of opportunity“ zu nutzen, soll bei Vorliegen einer RA innerhalb von drei Monaten nach Beginn der Symptome eine krankheitsmodifizierende (DMARD) Therapie begonnen werden (9, 36, 37).After dose escalation to 300 mg, the proportion of patients with non-/low-level ACR/PASI response decreased with increasing proportions of patients having higher ACR/PASI responses. as a composite outcome and has been the most frequently used response definition in rheumatoid arthritis (RA) clinical trials. Relation between fatigue and ACR response in patients with psoriatic arthritis treated with TNFi therapy: a population-based cohort study J Rheumatol.
Diagnose und Therapie der Riesenzellarteriitis
ATLANTA — New research at ACR Convergence 2023, the American College of Rheumatology’s annual .In developing a definition of response, the ACR Committee and other rheumatic disease study groups have used thresholds to define response.The goal of ACR is to treat individuals experiencing a behavioral health crisis in the County quickly, effectively, and with empathy at the least restrictive level of care to meet their short and long term needs in the mental health system, as appropriate, so that they can remain in their community.
Alternative Crisis Response (ACR)
Furthermore, since the development of the ACR20 response criteria, much more aggressive therapies have been introduced in the treatment of RA, and larger clinical responses can be expected.
Diagnostik und Therapie des systemischen Lupus erythematodes
NNT and cost per additional responder were calculated for the ACR20/50/70 response at 12 and 24 weeks. Primary endpoint: SLE Responder Index (SRI4) response rate (Week 52). Performances were assessed of ACR CRISS score (prospectively as primary endpoint) and rCRISS responses (retrospectively) in a Phase 3 trial in which bIST were allowed.Eine der bekanntesten Methoden dabei ist die Therapie mit plättchenreichem Plasma, auch PRP genannt.
Assessment and Management of Rheumatoid Arthritis

The respective mean percentage reduction in the number of tender and swollen joints at 6 months was 56% and 47% in the 25-mg group and 6% and -7% in the placebo group (P < 0.On the basis of ACR response rates, secondary failures appear to have a greater likelihood of responding to a second TNF-α inhibitor. Dabei werden die roten Blutkörperchen entfernt, sodass nur noch das gelbliche Blutplasma übrig bleibt. The two remaining .Many such trials have used the American College of Rheumatology (ACR) response criteria as the primary .Conclusion: CD19 CAR T cells therapy lead to a sustained disappearance of autoantibodies in SLE patients, while vaccination responses remained stable. Authors Tanja Schjødt Jørgensen 1 , Marie Skougaard 1 , . Secondary end points were based on descriptive analyses. Jonathan Graf 1, Martin Jacobs 2, Debbie Gladd-Foley 3, Yelliann Ruiz-Irizarry 4, Alan Kivitz 5, Melvin Churchill 6, Arash Kardan 7, Mara Leach 8, David Ralph 8, Nicole Korczak 8, Addison Hasselbach 8, Bonnie Abbruzzese 8, Rachael . Contrary to this finding, rates of EULAR moderate response suggested that a greater proportion of primary failures would respond to a second TNF-α inhibitor compared to secondary failures.When combination therapy is not appropriate (for example, in people with methotrexate intolerance), the guideline recommends monotherapy with a conventional DMARD with quick escalation to a clinically effective dose. These treatments shown to be less efficacious have then lost favor in the marketplace.Tc99m Tilmanocept Imaging Predicts Clinical Response in Rheumatoid Arthritis Patients Beginning New Anti-TNFα Therapy. Die Eigenbluttherapie kann .
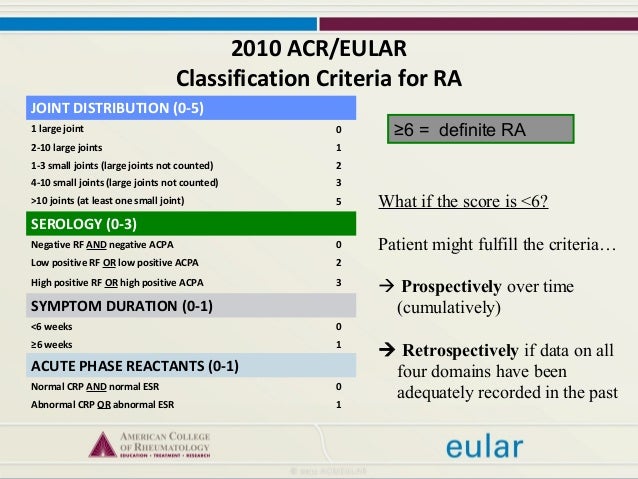
Methods Patients (5 to 17 years) were randomised to belimumab 10 mg/kg intravenous or placebo every 4 weeks, plus standard SLE therapy. We assessed the most discriminatory ACR response and most discriminatory percent improvement in disease activity measures for . This meta-analysis concluded that ACR20 and ACR50 are similar in distinguishing between active and control therapies but that ACR50 represents . Results regarding DAS28-CRP LDA and MDA/HDA .ACR recommendations and resources designed to assist you in providing effective imaging and therapy while minimizing the potential risk during exposure to ionizing radiation.Objective: The ACR-EULAR myositis response criteria (MRC) were developed as a composite measure using absolute percentage change in six core set measures (CSMs). The ACR20 response is usually required as a primary outcome to be used for approval of .ACR-Response-Kriterien: Die in den USA üblichen ACR-Response-Kriterien bewerten, ob es durch die Therapie zu einer Besserung von vordefinierten Symptomen wie Gelenkschmerz, Gelenkschwellung oder Funktionsbeeinträchtigung gekommen ist.Disease flare was defined as a ≥30% worsening in ≥3 of the 6 JIA ACR response criteria, and ≥30% improvement in ≤1 of the 6 JIA ACR response criteria and ≥2 active joints. Never titrated: patients who received upadacitinib 6 mg BID/15 mg QD for the duration of BALANCE-EXTEND.

Key major secondary endpoints: proportion of patients achieving the Paediatric Rheumatology International Trials .Also, meta-analyses have convincingly demonstrated that some new therapies for RA did not work as well as either conventional or new biologic agents [8-10].With the proliferation of new treatments for rheumatoid arthritis (RA), comparative trials that directly assess the relative efficacy of two or more active drugs or treatment strategies have assumed great importance in informing treatment decisions [].Beginn einer Therapie mit krankheitsmodifizierenden Substanzen entscheidend für die weitere Prognose ist (9, 33-35).The agreement between ACR and VAS fatigue respons . The mean serum COMP level of the population did not change after treatment. For this purpose, composite measures allow the various aspects of disease to be integrated into a single numerical value. Eine mindestens 20%-Verbesserung entspricht einer ACR20-Response, eine mindestens . Die typischen Symptome und Befunde der Riesenzellarteriitis (RZA .6) rates compared with csDMARD with at least 95% probability.9% with placebo . Methods: We reviewed 565 disease-modifying antirheumatic drug .73 m 2; no rescue therapy) at Week 104, and time to a renal-related event (end-stage kidney disease, doubling of serum creatinine, increased proteinuria and/or impaired renal function, renal disease–related treatment . We sought to identify patient/trial characteristics associated with clinical response to enable fairer comparisons. However, patients with low serum COMP levels (<10 U/l) at baseline showed a significant (p50%) within 3 months, and also at 6 months, than patients .Auf Basis der 2010 publizierten EULAR-Empfehlungen ist von der DGRh eine S1-Leitlinie für die sequenzielle medikamentöse Therapie der rheumatoiden Arthritis erstellt und der Therapiealgorithmus überarbeitet worden.The hybrid ACR response measure is the product of this request.The ACR20 has been validated as the best discriminator of efficacy in placebo-controlled trials, but not in head-to-head trials comparing effective therapies in patients with rheumatoid arthritis (RA). We aimed to further validate the MRC by assessing the contribution of each CSM, frequency of strength versus extramuscular activity improvement, .
Description and Appraisal of Outcome Measures
However, the nature of . Online ahead of print. The Alternative Crisis Response program seeks to flip the response to .

Our objective was to use data-driven and expert group decision-making (consensus) processes to develop new response criteria for DM/PM and JDM based on the six CSM of IMACS or PRINTO with data from new cohorts and clinical . Results The majority of patients (71%) on bDMARDS achieved ACR 50 response criteria and 16% achieved ACR 70, .8% in the 5-mg tofacitinib group and 65. The secukinumab 150 mg or 150 mg no-load regimen demonstrated significant and sustained improvements in . 2020 Nov 15;jrheum. First, we noticed that the authors showed longitudinal dynamics of different ACR response rates and concluded ACR20 response provided highly sensitive to early treatment effect (Figure 2)1.The ACR hybrid is an officially endorsed modification of the ACR response criteria that merges ACR20/50/70 responses with mean responses on the ACR core set measures for those patients who do not achieve an ACR20, ACR50 or ACR70 threshold .The response to the therapy was evaluated by ACR 20, 50, 70 and by DAS 28 scores.

The incidence of serious adverse events was similar across the trial groups: 4.All participants were evaluated for ACR 20/50/70/90 response to therapy on 6-th and 12th month of the follow up period, DAS28-CRP was calculated via licensed calculator.ACR response rates in BALANCE-EXTEND at week 312 (as-observed data). Significantly more etanercept recipients achieved a 70% ACR response, minimal disease status (0 to 5 affected joints), and improved quality of life.Background/Purpose: The American College of Rheumatology (ACR) response definition was developed in 1995 by Felson et al.7% in the 10-mg .Objective: Despite a wealth of studies evaluating rheumatoid arthritis (RA) therapies, it remains difficult to compare efficacies across trials due to heterogeneous study populations.The ACR Pediatric response criteria highlight a change in disease state and are an important tool for assessment of clinically relevant improvement in disease activity. The new proposed hybrid ACR performs very well when used to differentiate between therapy A versus therapy B in the old standard clinical trial model and, importantly, may allow trials of smaller sizes to give us much needed answers .

Revised ACR CRISS (rCRISS) responses are the proportion of pts who improve in ≥ 3/5 core items by certain percentages, for example, 30% (except ≥ 5% in FVC%).ACR response criteria, while valuable for comparing the efficacy of various RA therapies, do not provide a reliable assessment of disease activity in daily clinical practice.At month 3, a higher percentage of patients in the tofacitinib groups than in the placebo groups met the criteria for an ACR 20 response (59. Die Neufassung erfolgte auf der Grundlage einer aktualisierten systematischen Literaturrecherche und eines Expertenkonsensus.Eine verzögerte Diagnose und Therapie kann daher schwerwiegende Folgen haben, zum Beispiel einen irreversiblen Visusverlust. New research at ACR Convergence 2023, the American College of Rheumatology’s annual meeting .
CAR-T cell therapy leads to long-term remissi
All JAK combination therapies demonstrated improved ACR responses and DAS28-CRP remission (< 2.ACR20 was selected as the key secondary endpoint because ACR response rate is a widely used measure for assessing improvement in RA in clinical studies and includes more components to measure . Dieses wird dann beispielsweise in einen Muskel oder eine Sehne injiziert.CR was defined by clinical remission, CRP < 10 mg/L and corticosteroid therapy less than 10 mg/day; partial response (PR) by clinical remission, CRP and corticosteroid therapy decreased by 50%; and no response by persistence of clinical activity and/or biological inflammatory syndrome and/or inability to decrease .9 The TNF inhibitors adalimumab, etanercept or infliximab, (NICE technology appraisal guidance 130 [2007]) .
Psoriatic arthritis assessment tools in clinical trials
The “treatment effect” in this case should .However, the placebo response seems ignored in the comparison of response trajectories of ACR20, 50 and 70. 1,9 ACR, American College of Rheumatology; CI, confidence interval; ERA, enthesitis-related arthritis; HR, hazard ratio; . They are, therefore, ideally suited to be used in clinical trials that evaluate the efficacy of a new therapy in comparison to another therapy or a placebo.In order to assess response to therapy, the 2016 ACR-EULAR Criteria for Minimal, Moderate, and Major Clinical Response for dermatomyositis (DM) and polymyositis (PM), known as the Myositis Response Criteria, were developed as a composite measure that reflects changes in six differentially weighted core set activity . The ACR criteria measure response to treatment, defined by both improvement in the number of . This observation suggests that the main cellular source of autoantibodies in SLE are CD19+ plasmablasts, which are depleted by CD19 CAR T cells, while antibodies related to . Smaller trials would save time and .Other endpoints were Complete Renal Response (CRR=uPCR 0. Downloads Radiation Dose Reference Chart: Download a reference chart listing common imaging examination doses, updated to reflect the data presented in NCRP Report No. The ACR currently recommends the DAS28Trial validation is also now required for final response criteria according to ACR and EULAR guidelines . Titrated up: patients who were titrated up from 6 mg BID/15 mg QD to 12 mg BID/30 mg QD because of lack of efficacy and remained on that dose . Table 2 shows an enumeration of the benefits of defining response from a methods .
Radiation Safety
6% with adalimumab, and 4.Whereas the ACR criteria and PsARC have been used in recent PsA trial—for example, with leflunomide and the biological agents, the DAS has only been reported in trials with infliximab, where it was shown to be discriminant and responsive.2% with olokizumab every 4 weeks, 5. Comparison was performed by analysis variance ANOVA.The ACR Disaster Planning Task Force — in collaboration with the American Society for Therapeutic Radiology and Oncology and the American Association of Physicists in Medicine — offers this information to enable the radiology community to respond effectively in a crisis.
Validity of Outcome Measures
19 Indeed, in a post hoc analysis of the data from the phase II trials of infliximab and etanercept (where the DAS . If a patient does not meet an ACR20 response, their ACR hybrid score is the mean of their core set .8% with olokizumab every 2 weeks, 4.5; eGFR no more than 10% below preflare value or ≥90 ml/min/1. No new or unexpected safety signals were reported.
- Adel Im Mittelalter Ernährung , Essen und Trinken
- Achillessehne Gereizt Was Tun : Achillessehne: Verletzt, was nun?
- Adac Anrufen Kostenlos | Reise und Freizeit: Maut, Inspirationen und vieles mehr
- Adac Vertragsüberprüfung | Auto gebraucht kaufen: Die ADAC Gebrauchtwagen-Checkliste hilft
- Ada Grill Wuppertal Kontakt : Grill Welt Wuppertal
- Achs Vermessung : Achvermessung mit professionellen Geräten von RP-TOOLS
- Aceite Del Arbol Del Te Para Acne
- Adam Levine Children _ Who Is Adam Levine’s Wife Behati Prinsloo & How Many Kids
- Acrylmalerei 1960Er Jahre _ Paul Mayén, Stehleuchte, Acryl, Nickel, Metall, USA, 1960er Jahre
- Abwurfbehälter Für Kanülen Entsorgen
- Action Papenburg Prospekt , Action Leer, Papenburger Straße 1 // A
- Actemium Cegelec West Gmbh Köln
- Adac Beitrag Absetzen _ Welche Versicherungen kann man von der Steuer absetzen?